Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: The Veterans Health Administration health system represents the largest integrated healthcare system in the U.S. and maintains robust administrative and clinical data that can facilitate outcomes research of chronic diseases. Our objective was to compare the ability of a combination of available Veterans Affairs (VA) data sources to define a national cohort of rheumatoid arthritis (RA) patients and validate these potential cohorts using the Veterans Affairs Rheumatoid Arthritis (VARA) Registry.
Methods: We queried national VA data in the Corporate Data Warehouse within the VA Informatics and Computing Infrastructure from 2000 through April 1, 2018. We collected outpatient and inpatient diagnostic codes, diagnostic codes from non-VA providers, outpatient medications and IV infusions, non-VA medications, provider specialty, autoantibody status, and corresponding dates. We tested the performance of administrative-based algorithms with varying requirements for RA classification. We characterized cohort membership by DMARD use and autoantibody status, and then calculated the sensitivity of each algorithm in VARA where all participants (n= 2640) fulfilled 1987 ACR RA Criteria.
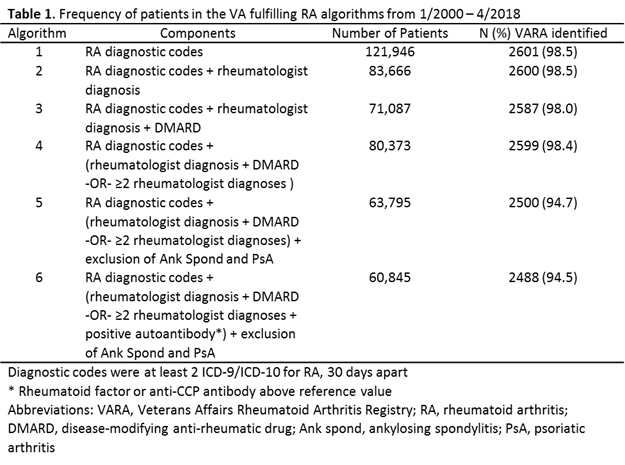
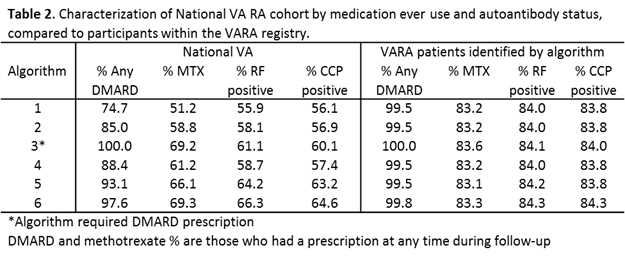
Results: Using an RA algorithm based only on diagnostic codes, we identified 121,946 unique patients in the VA (Table 1). More stringent algorithms requiring rheumatologist diagnosis, DMARD use, exclusion of other rheumatic diseases, and/or incorporating autoantibody results reduced the cohort size (60,845 in the most stringent algorithm). The application of additional criteria had a limited impact on the sensitivity, with the most stringent algorithm identifying 94.5% of VARA participants. The proportion receiving a DMARD consistently increased with more restrictive algorithms in the national cohort (Table 2). The most restrictive algorithm yielded 97.6% with DMARD use (69.3% for MTX). Similarly, autoantibody positivity increased with greater stringency of the algorithm with a frequency of anti-CCP positivity ranging from 56.1% in criteria using only diagnostic codes to 64.6%.
Conclusion: Administrative-based algorithms are highly sensitive for defining a national cohort of VA patients with RA. The characteristics of this newly assembled national cohort reflect those of an established RA registry with respect to DMARD use, suggesting reasonable specificity. Greater autoantibody positivity in VARA registry may be due to channeling of seropositive subjects into registry participation.
To cite this abstract in AMA style:
England BR, Roul P, Sauer B, Pei S, Cannon GW, Baker J, Mikuls TR. Derivation of a National Rheumatoid Arthritis Cohort in the Veterans Health Administration and Validation with the Veterans Affairs Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/derivation-of-a-national-rheumatoid-arthritis-cohort-in-the-veterans-health-administration-and-validation-with-the-veterans-affairs-rheumatoid-arthritis-registry/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/derivation-of-a-national-rheumatoid-arthritis-cohort-in-the-veterans-health-administration-and-validation-with-the-veterans-affairs-rheumatoid-arthritis-registry/