Session Information
Date: Sunday, November 13, 2016
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis - Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Denosumab is approved for treating postmenopausal women with osteoporosis at high risk for fracture. The placebo-controlled FREEDOM trial and its active-treatment Extension investigated the efficacy and safety of denosumab for up to 10 years. The lack of a long-term control group in the Extension, however, limits the ability to evaluate long-term efficacy. We used two approaches to put denosumab’s 10-year anti-fracture efficacy into perspective. First, we compared the 10‑year observed cumulative incidence of major osteoporotic (MOP; hip, clinical spine, forearm, or humerus) and hip fracture in subjects who completed the Extension with the 10-year fracture probability predicted at baseline by FRAX (a computer-based algorithm assessing fracture probability from clinical risk factors).1 The 10-year MOP fracture rate was also compared with that estimated for a hypothetical cohort of 10‑year placebo controls (virtual twins).
Methods: Subjects in this analysis received 10 years of denosumab (3 years FREEDOM; 7 years Extension; 60 mg Q6M), completed the 10-year visit, and missed ≤1 dose in FREEDOM and ≤1 dose in the Extension (n=1,278). Kaplan-Meier estimates of cumulative 10-year incidence of MOP and hip fracture were determined. Ten-year probability of fracture predicted by FRAX (calculated with femoral neck BMD) at FREEDOM baseline was also estimated. Rate of MOP fracture in a hypothetical cohort of 10-year placebo controls (virtual twins) was estimated using a previously described simulation method and baseline characteristics identical to the 10-year denosumab completer group.2,3
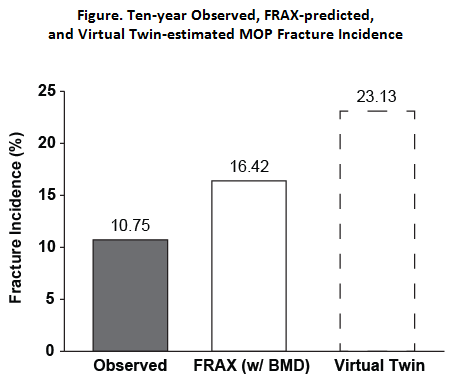
Results: The observed cumulative 10-year fracture incidence (95% CI) was lower than the 10‑year mean (SD) fracture probability predicted by FRAX for both MOP (10.75% [9.05%–12.46%] vs 16.42% [9.06%]; Figure) and hip (1.17% [0.58%–1.76%] vs 6.14% [6.52%]) fractures. The observed cumulative 10-year MOP fracture incidence was also significantly lower than the estimated virtual twins fracture rate (10.75% [9.05%–12.46%] vs 23.13% [17.76%–28.87%]; RR=0.49 [0.36–0.64]).
Conclusion: Fracture incidence with 10 years of denosumab treatment in postmenopausal women with osteoporosis was lower than the 10-year probability predicted by FRAX for both MOP and hip fractures. It was also lower than the fracture rate estimated in a hypothetical cohort of 10-year placebo controls for MOP fracture. These data support the long-term efficacy of denosumab in reducing MOP and hip fractures. References: 1https://www.shef.ac.uk/FRAX/index.aspx; 2Vittinghoff Stat Med 2010; 3Papapoulos Osteoporos Int 2015
To cite this abstract in AMA style:
Siris E, Pannacciulli N, Miller P, Lewiecki E, Chapurlat R, Jódar-Gimeno E, Daizadeh N, Wagman R, Kanis J. Denosumab Treatment for 10 Years in Postmenopausal Women with Osteoporosis Was Associated with Substantially Lower Fracture Incidence Relative to Their Baseline FRAX-Predicted Probability [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/denosumab-treatment-for-10-years-in-postmenopausal-women-with-osteoporosis-was-associated-with-substantially-lower-fracture-incidence-relative-to-their-baseline-frax-predicted-probability/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/denosumab-treatment-for-10-years-in-postmenopausal-women-with-osteoporosis-was-associated-with-substantially-lower-fracture-incidence-relative-to-their-baseline-frax-predicted-probability/