Background/Purpose: Cortical bone loss is a major determinant of increased fracture risk. Denosumab (DMAb) has been shown to increase BMD at sites of cortical bone, including the radius, a skeletal site not responsive to most osteoporosis treatments. Here, we evaluated changes over time in radius BMD and wrist fracture incidence during 3 years of placebo (Pbo) and up to 5 subsequent years of DMAb therapy in FREEDOM and its Extension (EXT).
Methods: We evaluated 2207 women who received Pbo during FREEDOM (3 years) and enrolled in the EXT to receive DMAb 60 mg Q6M (cross-over group); all women received daily calcium and vitamin D. A subset of these women (n=115) participated in a distal radius DXA substudy and were evaluated at baseline and during FREEDOM and EXT. Analysis of mean percentage changes in BMD over time from FREEDOM and EXT baselines consisted of a repeated measure model. Wrist fracture rates (per 100 subject-years), rate ratios, and 95% CI were computed.
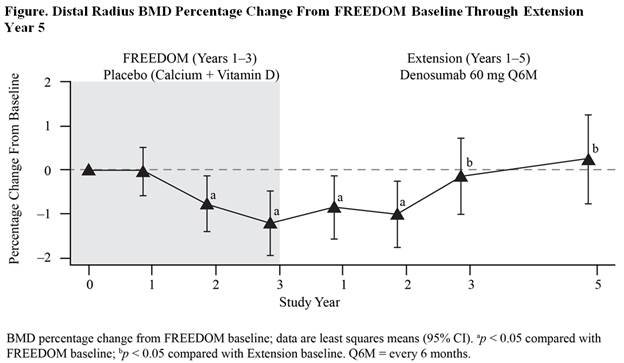
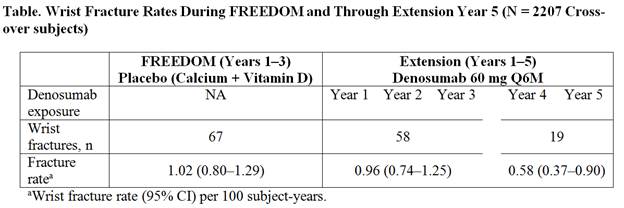
Results: At FREEDOM baseline, the mean (SD) 1/3 radius T-score was –2.53 (1.18). During FREEDOM, daily calcium and vitamin D alone was associated with a progressive and significant loss of BMD at the 1/3 radius (–1.2%); however, during EXT, DMAb halted and reversed bone loss (Figure). With 5 years of DMAb treatment, a significant gain in BMD (1.5% at EXT Year 5) was observed, compared with EXT baseline. The wrist fracture rate during the Pbo period in FREEDOM was 1.02 (0.80–1.29) per 100 subject-years. During the first 3 years of EXT, BMD recovered to the original baseline levels in response to DMAb and the wrist fracture rate remained comparable to the FREEDOM Pbo rate (Table); with 2 additional years of DMAb treatment, BMD increased further and the wrist fracture rate declined to levels significantly lower than the FREEDOM Pbo rate (rate ratio=0.57, 95% CI=0.34–0.95; p=0.03).
Conclusion: In untreated women with postmenopausal osteoporosis, cortical bone density at the radius declined significantly. DMAb treatment for 3 years fully reversed this bone loss, and 2 additional years of treatment resulted in further BMD gains that translated to significantly lower wrist fracture rates, highlighting the clinical importance of reversing cortical bone loss.
Disclosure:
J. Bilezikian,
NIH, Amgen Inc., NPS,
2,
Columbia University,
3,
Merck, Amgen Inc., NPS, Lilly, Johnson&Johnson,
5,
Elsevier Press,
7;
C. Benhamou,
Amgen Inc., MSD, Servier,
2,
Amgen Inc., Lilly, Novartis, Roche, Rottapharm, Servier,
5;
C. Lin,
Amgen Inc.,
1,
Amgen Inc.,
3;
J. Brown,
Actavis, Amgen Inc., Eli Lilly, Merck, Novartis,
2,
Amgen Inc., Eli Lilly,
5,
Amgen Inc., Eli Lilly,
8;
N. Daizadeh,
Amgen Inc.,
1,
Amgen Inc.,
3;
P. Ebeling,
Amgen Inc., GSK, Eli-Lilly, Merck, Novartis,
2,
GSK, Amgen Inc., Merck,
5;
A. Fahrleitner-Pammer,
Roche,
2,
Amgen Inc., GSK, Eli Lilly, Servier, Pfizer,
8;
E. Franek,
Amgen Inc., Novartis,
5,
Amgen Inc., Eli Lilly, Novartis, Servier, TEVA,
8;
N. Gilchrist,
None;
P. Miller,
Amgen Inc., Merck, Lilly, Takeda, Radius, Boehringer, Novo Nordisk,
2,
Merck, Amgen Inc., Lilly,
5;
J. Simon,
AbbVie Inc., Actavis, PLC., EndoCeutics Inc., Novo Nordisk, Novogyne, Palatin Technologies, Teva Pharmaceutical Industries Ltd,
2,
AbbVie Inc., Actavis, PLC., Amgen Inc., Apotex Inc., Ascend Therapeutics, Depomed Inc., Everett Laboratories Inc., Lupin Pharmaceuticals, TherapeuticsMD, Meda Pharmaceuticals Inc., Merck and Co Inc., Novartis Pharmaceuticals Corp,,
5,
Amgen Inc., Eisai Inc., Merck, Noven Pharmaceuticals Inc., Shionogi Inc., Teva Pharmaceutical Industries Ltd,
8;
I. Valter,
None;
C. Zerbini,
Pfizer, Lilly, Merck, Amgen Inc., Novartis,
2,
MSD, Lilly, Pfizer, Servier,
5,
Pfizer, Lilly, Servier,
8;
C. Libanati,
Amgen Inc.,
1,
Amgen Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/denosumab-restores-cortical-bone-loss-at-the-distal-radius-associated-with-aging-and-reduces-wrist-fracture-risk-analyses-from-the-cross-over-group-in-the-extension-of-the-denosumab-pivotal-fracture/