Session Information
Session Type: Abstract Submissions (ACR)
Denosumab Leads to Significantly Greater Increases in Bone Mineral Density Than Ibandronate and Risedronate in Postmenopausal Women at High Risk for Fracture Who Were Previously Treated With an Oral Bisphosphonate
Background/Purpose: Low bone mineral density (BMD) is an important and modifiable risk factor for fracture in postmenopausal women with osteoporosis. Denosumab (DMAb), which has a unique mechanism of action, has demonstrated a stronger relationship between BMD increases and antifracture efficacy (new or worsening vertebral and nonvertebral fracture) than bisphosphonate (BP) therapies. There is a clinical need to treat subjects who remain at higher risk for fracture despite current BP therapy. In 2 open-label studies, DMAb significantly increased BMD and decreased bone turnover markers vs a monthly oral BP, specifically ibandronate (IBN; 150 mg, single dose once monthly) or risedronate (RIS; 75 mg on 2 consecutive days once monthly), in subjects previously treated with, though suboptimally adherent to, a BP. Here we evaluate the effects of DMAb vs a monthly oral BP (IBN and RIS) to increase BMD in a subset of subjects at higher risk for fracture.
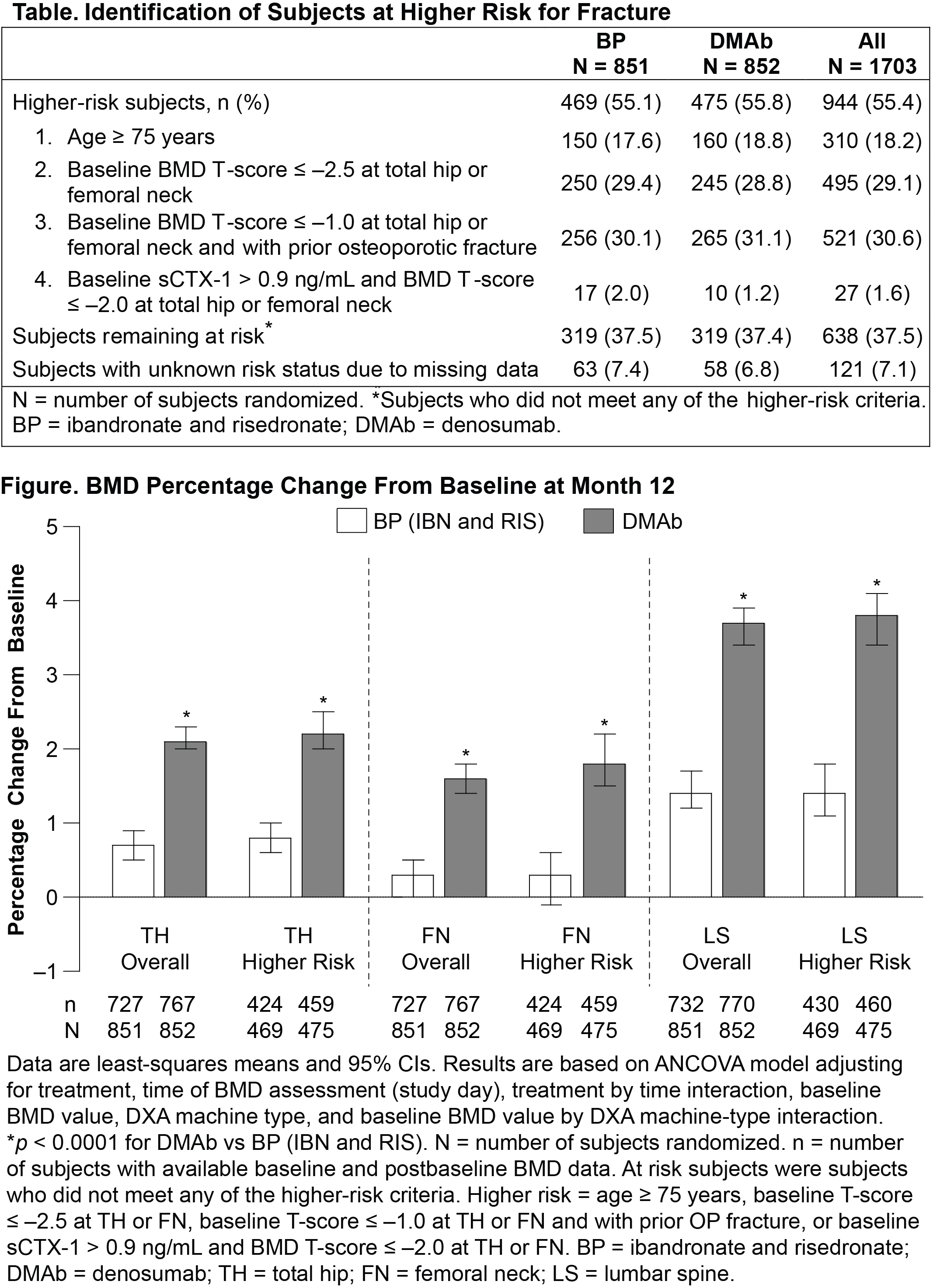
Methods: Both studies were multicenter, randomized, open-label, parallel-group designs in which postmenopausal women ≥ 55 years were randomized 1:1 to DMAb 60 mg subcutaneously every 6 months or a BP 150 mg orally every month for 12 months. In this combined post-hoc analysis, higher-risk subjects were identified by meeting ≥ 1 risk criteria (advanced age, low BMD, prior osteoporotic fracture, high baseline sCTX-1; Table) and the BMD percentage change from baseline in total hip, femoral neck, and lumbar spine at month 12 was calculated.
Results: Subjects from these 2 studies (852 DMAb; 851 BP) had a mean (SD) age of 67 (7.4) years, and mean (SD) T-score at the total hip, femoral neck, and lumbar spine of –1.7 (0.8), –2.0 (0.7), and –2.4 (1.0), respectively. For subjects at higher risk for fracture, DMAb significantly increased BMD at 12 months compared with a BP (IBN and RIS) at the total hip (2.2% vs 0.8%, respectively), femoral neck (1.8% vs 0.3%), and lumbar spine (3.8% vs 1.4%) (p < 0.0001 for all; Figure). These results are consistent with the overall study population (treatment-by-risk subgroup interaction p > 0.05). In general, AEs and SAEs were similar between DMAb and the comparator BP group.
Conclusion: For subjects previously or suboptimally treated with a BP who remain at higher risk for fracture, transitioning to DMAb led to significantly greater increases in BMD at month 12 compared with cycling to another BP. These results in higher-risk subjects are consistent with those obtained in the overall population and support DMAb as an alternative therapeutic option for women at higher risk for fracture.
Disclosure:
J. P. Brown,
Amgen Inc., Eli Lilly, Merck, Novartis, sanofi-aventis, Warner Chilcott,
5,
Abbott, Amgen Inc., Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, sanofi-aventis, Servier, Warner Chilcott, Takeda,
2,
Amgen Inc., Eli Lilly, Novartis, Merck,
9;
M. A. Bolognese,
Vivus, Lilly, NOF,
5,
Amgen Inc., Lilly, Genentech,
2;
P. R. Ho,
Amgen Inc.,
1,
Amgen Inc.,
3;
J. Hall,
Amgen Inc.,
1,
Amgen Inc.,
3;
C. Roux,
MSD, AMGEN Inc., NOVARTIS, LILLY,
5,
BONGRAIN, LILLY, MSD,
2;
H. G. Bone,
Amgen Inc., Merck, Tarsa, Novartis ,
5,
Amgen Inc., Merck, Tarsa, Novartis, Lilly,
2;
S. Bonnick,
Amgen Inc., Novartis,
7;
J. van den Bergh,
Amgen Inc., MSD,
5,
Eli Lilly, MSD, Amgen Inc., Will Pharma,
8;
I. Ferreira,
Amgen Inc.,
1,
Amgen Inc.,
1;
P. Ghelani,
Amgen Inc.,
1,
Amgen Inc.,
3;
P. Dakin,
Amgen Inc.,
1,
Amgen Inc.,
3;
R. B. Wagman,
Amgen Inc.,
1,
Amgen Inc.,
3;
C. Recknor,
Novartis, Eli Lilly, Amgen Inc. ,
5,
Novartis, Warner Chilcott,
8,
Ion Med Systems,
9,
Medi ,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/denosumab-leads-to-significantly-greater-increases-in-bone-mineral-density-than-ibandronate-and-risedronate-in-postmenopausal-women-at-high-risk-for-fracture-who-were-previously-treated-with-an-oral-b/