Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Given the unmet clinical needs in systemic lupus erythematosus (SLE), including poor disease control and drug toxicities, new therapies are needed. In a phase 2, randomized, placebo-controlled, double-blind study (JAHH), once-daily baricitinib (bari) resulted in significant clinical improvement in patients (pts) with active SLE versus (vs.) placebo (PBO). Bari inhibits Janus Kinase (JAK)1 and JAK2 signaling, and in turn may affect signal transducer and activator of transcription (STAT)1, STAT2 and STAT4 pathways. Therefore, bari has the potential to simultaneously impact several pro-inflammatory immune cytokines implicated in the pathogenesis of SLE, including interferon (IFN)-α, IFN-γ, interleukin (IL)-6, IL-12, and IL-23. The objectives of the current study were: 1) to examine baseline serum cytokines in the JAHH phase 2 clinical trial for correlations with clinical or immunologic assessments; 2) to determine if changes in serum cytokine levels were associated with bari treatment.
Methods: Pts enrolled in the JAHH phase 2 trial received daily treatment with PBO, bari 2 mg, or bari 4 mg through Week 24. Serum samples were collected at baseline (Week [Wk] 0), Wk 12, and Wk 24) from SLE pts (n=270) and 50 sex- and age-matched controls. Samples were analyzed for: IL-2, IL-3, IL-5, IL-6, IL-10, IL-17A, IL-21, IL-12/23p40, IL-12p70, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-α and IFN-γ using ultrasensitive quantitative assays. IFN gene signature, autoantibodies, complement component 3 and 4 (C3 and C4) were measured as previously described.1
Results: At Wk 0, serum IL-17A, IL-12/23p40, IL-6, IFN-γ and IFN-α were readily detectable. IL-12/23p40 was detectable in 100% of pts vs. 100% of controls, IFN-γ in 89% of pts vs. 66% of controls, IL-6 in 53% of pts vs. 12% of controls and IFN-α in 41% of pts vs. 2% of controls; detection of serum IL-2, GM-CSF, IL-5, IL-10 and IL-17A was variable (Figure 1). At baseline (Wk 0), IL-12/23p40 was positively correlated with SLE disease activity index and IFN gene signature, and negatively correlated with serum C4. IL-6 was positively correlated with joint swelling, joint tenderness, IFN-γ and C3. Serum IFN-α was positively correlated with serum IFN-γ, antibodies to Smith and ribonucleoprotein antigens (anti-Sm and anti-RNP), and the IFN gene signature (Figure 2). Treatment with bari 4 mg (Figure 1B) significantly decreased serum IL12/23p40 and IL-6 cytokine levels at Wk 12 (p< 0.05) but not serum IFN-α or IFN-γ levels (Figure 1B).
Conclusion: Bari 4 mg treatment was associated with statistically significant decrease of serum IL-12/23p40 and IL-6 at Week 12 which continued through Week 24. Serum IFN-α or IFN-γ were not reduced with bari treatment. Thus, bari 4 mg simultaneously
impacted multiple pro-inflammatory cytokines implicated in the pathogenesis of SLE.
Reference:
1. Hoffman RW, et al. Arthritis Rheumatol. 2017;69(3):643-654.
 *p = 0.015; **p = 0.001; Bari=baricitinib; IFN=interferon; IL=interleukin; LLOQ=lower limit of quantification; PBO=placebo; SLE=systemic lupus erythematosus.
*p = 0.015; **p = 0.001; Bari=baricitinib; IFN=interferon; IL=interleukin; LLOQ=lower limit of quantification; PBO=placebo; SLE=systemic lupus erythematosus.
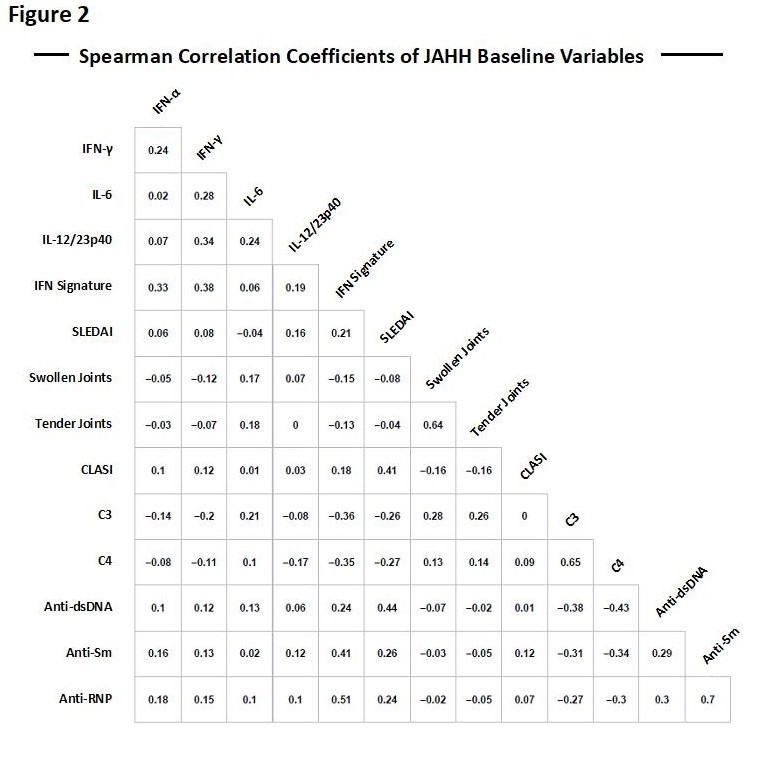 Anti-dsDNA=Anti-double stranded DNA; Anti-RNP=Anti ribonucleoprotein; Anti-Sm=Anti-Smith; CLASI=cutaneous lupus erythematosus disease area and severity index; C3=complement component 3; C4=complement component 4; IFN=interferon; IL=interleukin; SLEDAI=systemic lupus erythematosus disease activity index.
Anti-dsDNA=Anti-double stranded DNA; Anti-RNP=Anti ribonucleoprotein; Anti-Sm=Anti-Smith; CLASI=cutaneous lupus erythematosus disease area and severity index; C3=complement component 3; C4=complement component 4; IFN=interferon; IL=interleukin; SLEDAI=systemic lupus erythematosus disease activity index.
To cite this abstract in AMA style:
Dörner T, Tanaka Y, Petri M, Smolen J, Wallace D, Crowe B, Dow E, Higgs R, Rocha G, Benschop R, Silk M, de Bono S, Hoffman R, Fantini D. Delineation of a Proinflammatory Cytokine Profile Targeted by Janus Kinase 1/2 Inhibition Using Baricitinib in a Phase 2 Systemic Lupus Erythematosus Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/delineation-of-a-proinflammatory-cytokine-profile-targeted-by-janus-kinase-1-2-inhibition-using-baricitinib-in-a-phase-2-systemic-lupus-erythematosus-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/delineation-of-a-proinflammatory-cytokine-profile-targeted-by-janus-kinase-1-2-inhibition-using-baricitinib-in-a-phase-2-systemic-lupus-erythematosus-trial/
