Session Information
Date: Tuesday, October 28, 2025
Title: Plenary III (1722–1727)
Session Type: Plenary Session
Session Time: 8:00AM-8:15AM
Background/Purpose: Current guidelines recommend using hydroxychloroquine (HCQ) dose ≤5.0 mg/kg for managing SLE. However, 6-fold higher SLE flares, including those requiring hospitalizations, are noted with HCQ dose of ≤5mg/kg vs. >5mg/kg (Jorge, 2022). Moreover, clear guidance on adjusting HCQ dose in chronic kidney disease (CKD) is lacking. HCQ blood level monitoring could better guide precise HCQ dosing based on patient risk factors. Leveraging data from diverse SLE cohorts, we aimed to study the upper threshold to define a therapeutic range for HCQ blood levels to support clinical use and inform dose adjustments.
Methods: Data were pooled from the SLICC Inception Cohort (n=660, 33 centers in 11 countries in North America, Europe, Asia), one longitudinal prevalent SLE registry (n=269, Wisconsin, USA), and three previously published studies of HCQ levels in SLE (n=1081, centralized in Paris, France). HCQ levels (T0) at first visit for the registry/cohort were measured in whole blood or serum; HCQ serum levels were converted to HCQ whole blood equivalent using the validated conversion factor of dividing by 0.53. The primary outcome was HCQ-related systemic toxicity (cardiac, retinal, muscle) at the last follow-up (T last visit), identified from clinical reports. SLE Disease Activity Index (SLEDAI-2K) at T0 was abstracted to identify active SLE defined as SLEDAI-2K ≥6 (Secondary outcome). Using mixed regression analysis, we first identified a HCQ blood level (T0) predictive of higher HCQ toxicity (T last visit). Next, we tested if this upper threshold was supratherapeutic (no further risk reduction for active SLE). Finally, we examined associations between CKD stage ≥3 and supratherapeutic/toxic HCQ blood levels at T0.
Results: Among 1842 patients, 4.4% had toxicity (85% retinopathy, 9% cardiomyopathy, 6% myopathy). Odds of toxicity were 1.9-fold higher with blood levels >1150 ng/mL (Fig. 1A, Table 1A), and 1.04-fold higher with cumulative HCQ dose per 100g increase (Table 1A). Using 750-1150 ng/ml as a reference range, levels < 750 ng/mL were associated with 1.4 fold higher odds of active SLE (Table 1B), while levels >1150 ng/mL were associated with a saturation in therapeutic effect, indicating supratherapeutic levels (Fig. 1B, Table 1B). Patients on HCQ dose < 5mg/kg had 1.9-fold higher odds of active SLE (Table 1B); 52% of patients on ≤5mg/kg dose had subtherapeutic levels (< 750 ng/ml), and 18% supratherapeutic levels ( >1150 ng/mL).Patients with CKD stage ≥3 had 2.3-fold higher odds of having supratherapeutic/toxic levels ( >1150 ng/mL) (Table 2A). These associations remained significant even in patients on HCQ dose ≤5mg/kg (OR=1.94; Table 2B).
Conclusion: HCQ whole blood levels >1150 ng/mL predicted 2-fold higher risk of HCQ-related toxicity (confirming findings in Petri et al 2020), while levels < 750 ng/mL were associated with 1.4-fold higher active SLE risk. Even with HCQ dose ≤5mg/kg, 52% had subtherapeutic vs. 18% had supratherapeutic levels risking active SLE vs. toxicity, while those with CKD stage ≥3 had supratherapeutic/toxic levels ( >1150 ng/mL). These findings establish the clinical significance of HCQ blood level monitoring to guide precise and optimal HCQ use to balance safety and efficacy in SLE.
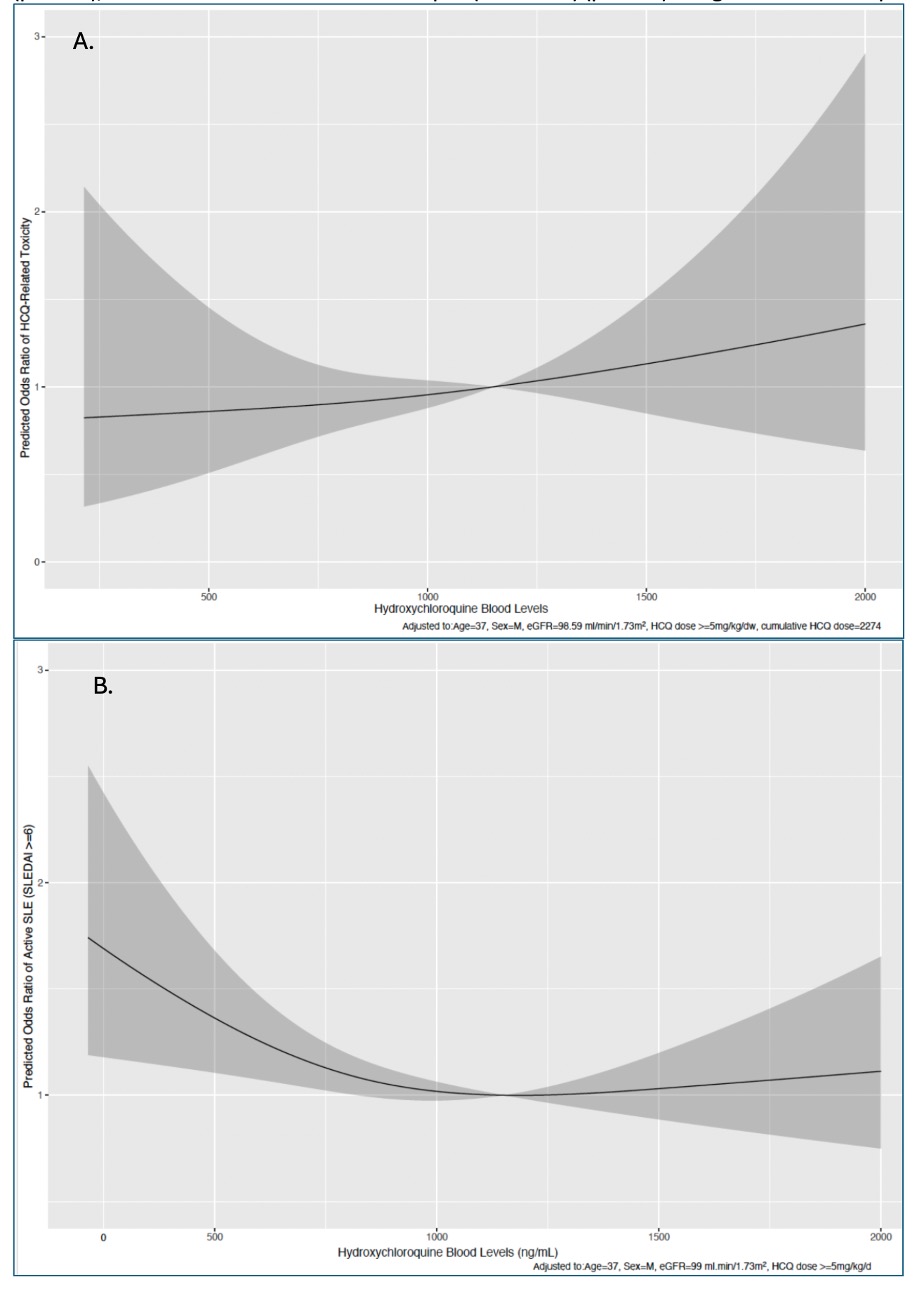 Fig. 1. Adjusted restricted cubic splines to show associations between: HCQ blood levels and toxicity (panel A), and HCQ blood levels and active lupus (SLEDAI ≥6) (panel B) using data from 1842 patients
Fig. 1. Adjusted restricted cubic splines to show associations between: HCQ blood levels and toxicity (panel A), and HCQ blood levels and active lupus (SLEDAI ≥6) (panel B) using data from 1842 patients
.jpg) Table 1A. Multivariable logistic regression analysis showing factors (T0) associated with HCQ toxicity (T last visit) of HCQ using data from different populations (n=1842); Table 1B. Multivariable logistic regression analysis showing factors (T0) associated with clinical response (active SLE defined as SLEDAI ≥6) at T0 with HCQ use using data from different populations (n=1842)
Table 1A. Multivariable logistic regression analysis showing factors (T0) associated with HCQ toxicity (T last visit) of HCQ using data from different populations (n=1842); Table 1B. Multivariable logistic regression analysis showing factors (T0) associated with clinical response (active SLE defined as SLEDAI ≥6) at T0 with HCQ use using data from different populations (n=1842)
.jpg) Table 2A. Multivariable logistic regression analysis showing factors (T0) associated with supratherapeutic (or toxic) whole blood levels, >1150 ng/mL, at T0 across all patients (n=1842); Table 2B. Multivariable subgroup analysis showing factors (T0) associated with supratherapeutic (or toxic) whole blood levels, >1150 ng/mL, at T0 including only patients on dose ≤5mg/kg (n=781)
Table 2A. Multivariable logistic regression analysis showing factors (T0) associated with supratherapeutic (or toxic) whole blood levels, >1150 ng/mL, at T0 across all patients (n=1842); Table 2B. Multivariable subgroup analysis showing factors (T0) associated with supratherapeutic (or toxic) whole blood levels, >1150 ng/mL, at T0 including only patients on dose ≤5mg/kg (n=781)
To cite this abstract in AMA style:
Garg S, Blanchet B, Nguyen Y, Hollnagel F, Clarke A, Petri M, Urowitz M, Hanly J, Gordon C, Bae S, Romero-Diaz J, Sanchez-Guerrero J, Clarke A, Bernatsky S, Wallace D, A. Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Bruce I, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jönsen A, Alarcón G, van Vollenhoven R, Aranow C, Inanc M, mackay M, Ruiz-Irastorza G, Lim S, Inac M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Buyon J, Chezel J, Puszkiel A, Costedoat-Chalumeau N. Defining Safe Hydroxychloroquine Blood Levels: Time to Switch to Precision Monitoring for Optimized Lupus Care [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/defining-safe-hydroxychloroquine-blood-levels-time-to-switch-to-precision-monitoring-for-optimized-lupus-care/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-safe-hydroxychloroquine-blood-levels-time-to-switch-to-precision-monitoring-for-optimized-lupus-care/
