Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes I: Assessment Tools
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: In axial Spondyloarthritis (axSpA) it is recommended to assess global functioning and health, a concept close to health-related quality of life (HR-QoL), using the ASAS Health Index (ASAS-HI), a patient-reported outcome measure [1]. Impairment status of poor global health (GH) has been defined as an ASAS-HI score of ≥ 12/17 [1]. However, the ASAS-HI is a recent outcome measure and is not always available in existing datasets. Our objective was to explore the link between ASAS-HI and a frequently used measure of GH and utility, the EuroQol-5D-3L (EQ-5D) and to explore the possibility of defining a cut-off value for EQ-5D, corresponding to the ASAS-HI cut-off of 12 points.
Methods: This was a reanalysis of a cross-sectional observational study: PERSPA, analyzing the subgroup of patients fulfilling ASAS criteria for axSpA, and with available EQ-5D and ASAS-HI scores [2]. The ASAS-HI is a specific composite measure for GH in axSpA, scored 0-17, with a validated cut-off of ≥ 12/17 for poor health status [1]. The EQ-5D is a generic score for utility and HR-QoL, with an index value between 1 (perfect health) and 0 or negative (health state equivalent to death or worse) [3] – here we reversed the scores to align with better health being lower scores (as for the ASAS-HI). Distributions of EQ-5D and ASAS-HI were visually compared with assessment of normality by Shapiro-Wilk test. The correlation between ASAS-HI and EQ-5D (Spearman) was computed. Patients were grouped into deciles based on EQ-5D and ASAS-HI scores, and weighted kappa was calculated. Then, to determine a cut-off for EQ-5D corresponding to an ASAS-HI ≥ 12, a ROC curve of EQ-5D according to ASAS-HI category was obtained; the Youden index for EQ-5D that maximizes sensitivity and specificity was then determined.
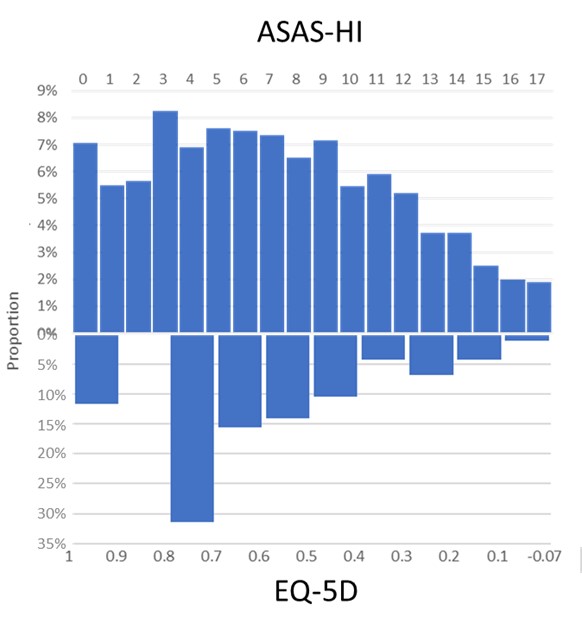
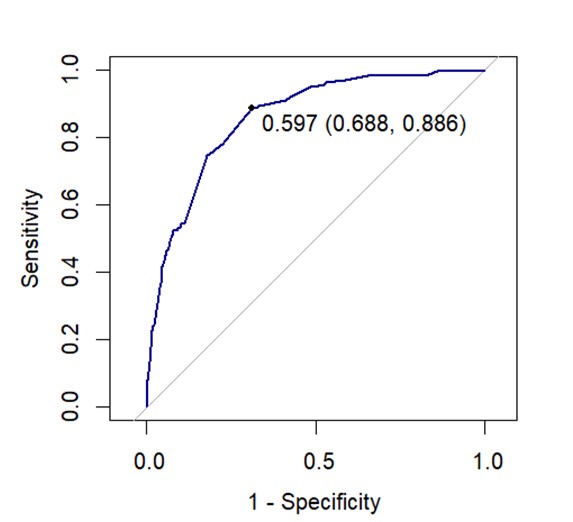
Results: In 2651 patients (mean age 42.0 years, 66.5% men), GH was generally moderate to good using both the ASAS-HI and EQ-5D: mean ASAS-HI was 7.05 (SD 4.49) (median 7.00) and mean EQ-5D was 0.63 (SD 0.23) (median 0.65). The distributions appeared similar overall though neither distribution was normal (Shapiro-Wilk p< 0.001) (Figure 1). ASAS-HI had a high negative correlation with EQ-5D (r = -0.71, p < 0.001), however the agreement between deciles was moderate (weighted kappa = 0.51). Using the published cutoff of 12 for ASAS-HI, we established that 448 (16.9%) patients had severe impairment of GH. The ROC curve showed a satisfactory link between the 2 scores: area under the curve was 0.86 (Figure 2). The threshold of 0.597 for EQ-5D maximized both sensitivity (0.89) and specificity (0.69) against the ASAS-HI cut-off of 12.
Conclusion: The EQ-5D, a widely validated, widely-available generic score to assess utility, can be used to assess overall GH in axSpA, which is usually assessed through the ASAS-HI. In a large group of patients, we have shown that the two outcome measures are linked, with a high correlation but no full overlap. Furthermore, we propose an EQ-5D threshold corresponding to the published ASAS-HI threshold for severe impairment in GH. These results may be useful for comparing GH when only one of the outcome measures is available.
(1) Kiltz U, et al., Ann Rheum Dis 2018 ;77 :1311-7
(2) López-Medina C, et al., RMD Open 2021;7:e001728.
(3) https://euroqol.org
To cite this abstract in AMA style:
Drouet J, López Medina C, Molto A, Granger B, Fautrel B, Gaujoux Viala C, Kiltz U, Dougados M, Gossec L. Defining Poor Global Functioning and Health in Axial Spondyloarthritis, Using the EQ-5D-3L Questionnaire, When the ASAS-Health Index Is Not Available Is Feasible. an Analysis of 2651 Patients from the PERSPA Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/defining-poor-global-functioning-and-health-in-axial-spondyloarthritis-using-the-eq-5d-3l-questionnaire-when-the-asas-health-index-is-not-available-is-feasible-an-analysis-of-2651-patients-from-the/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-poor-global-functioning-and-health-in-axial-spondyloarthritis-using-the-eq-5d-3l-questionnaire-when-the-asas-health-index-is-not-available-is-feasible-an-analysis-of-2651-patients-from-the/