Session Information
Date: Sunday, October 21, 2018
Title: 3S111 ACR Abstract: Spondyloarthritis Incl PsA–Clinical II: PsA Epidemiology (964–969)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
The Psoriatic Arthritis (PsA) Impact of Disease questionnaire (PsAID12) score allows an assessment of patient-important symptoms and life impact in PsA. It is provisionally recommended as core outcome measure by OMERACT (ref). To date, no cutoffs have been established to help clinicians interpret its results. The objective was to develop such cutoffs for PsAID12 corresponding to disease activity states (i.e. remission (REM), low disease activity (LDA) or moderate disease activity).
Methods:
ReFlap (NCT03119805) is an ongoing cross-sectional study in 14 countries of consecutive adult patients with definite PsA and more than 2 years of disease duration. The PsAID12 (0-10 score where 0 is no impact) was collected. Disease activity was defined using the following external anchors: (a) Disease Activity in PSoriatic Arthritis (DAPSA) score (cutoffs of ≤4, ≤14, ≤28 for REM, LDA and moderate disease activity, respectively) (b) Very Low Disease Activity (VLDA) / Minimal Disease Activity (MDA) scores (cutoffs of 7/7 and 5/7 criteria met for REM and LDA, respectively) (c) single questions for patient and physician-defined REM and LDA. For each level of disease activity and for each external anchor, cutoffs of PsAID12 were calculated using Youden’s index on Receiver Operating Characteristic (ROC) curve analyses and the 75th percentile method. Finally, author consensus was sought on final proposed cutoffs.
Results:
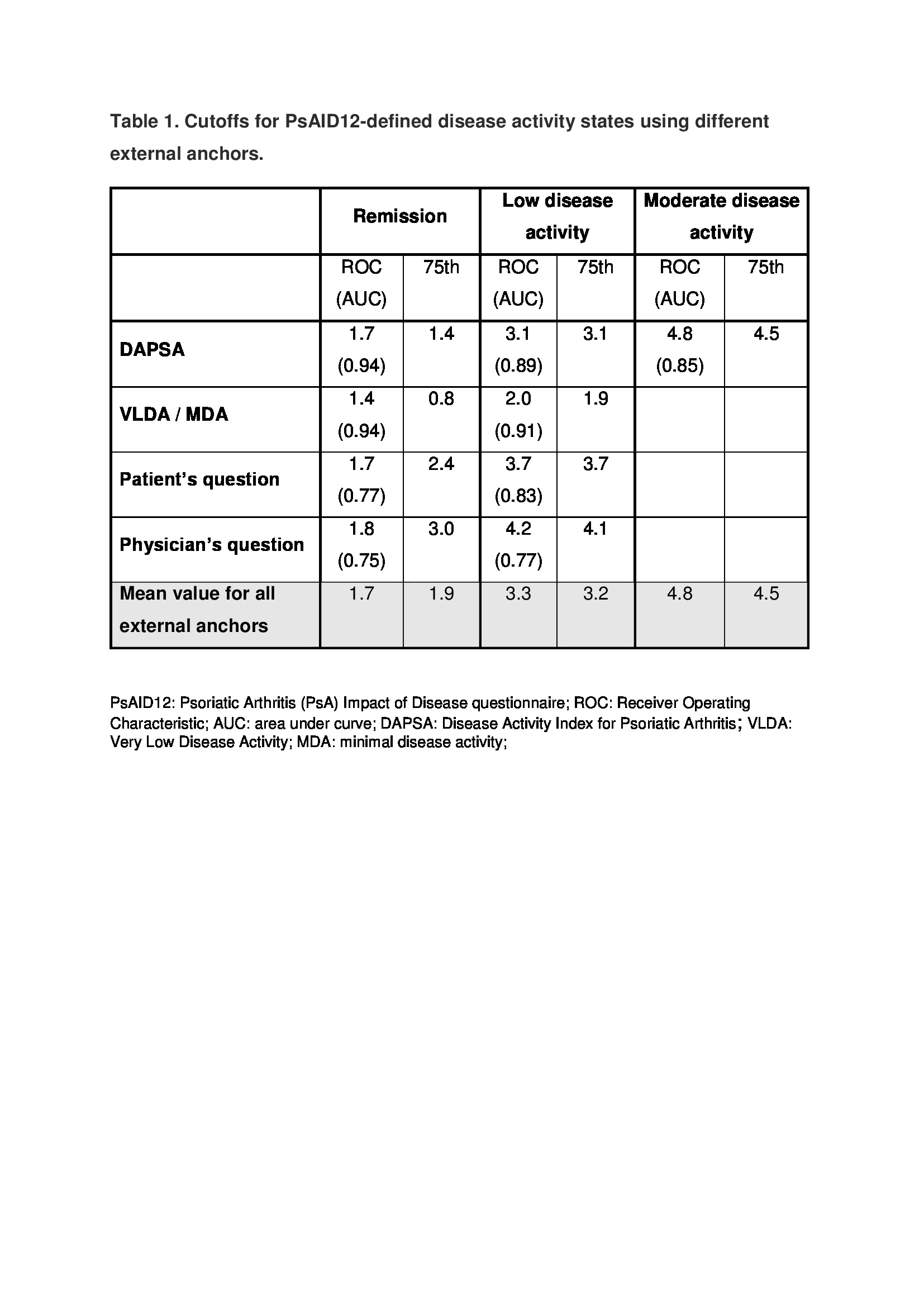
Of 466 patients, 436 were analyzed: 218 (50.8%) were men, mean age (standard deviation) was 53.6 (12.7) years, mean disease duration was 11.3 (8.3) years, 62.3% were taking conventional synthetic DMARDs and 60.1% a biologic. Mean PsAID12 was 3.4 (2.5). The frequency of REM varied from 12.8% (VLDA) to 38.5% (Physician REM question) and of LDA from 25.5% (MDA) to 43.1% (Patient LDA question). PsAID functioned well against the external anchors as indicated by high areas under the ROC curves (range, 0.75-0.94). The VLDA and MDA criteria were more difficult to reach than the DAPSA REM and LDA cutoffs, reflected by much lower cutoffs for the PsAID12 score (Table 1). PsAID12 cutoffs varied between 1.7-1.9, 3.2-3.3 and 4.5-4.8 for REM, LDA and moderate disease activity, respectively (Table 1). Proposed final cutoffs of PsAID12 are <2 for REM, <=3 for LDA and <5 for moderate disease activity.
Conclusion:
It was possible to define cutoffs for PsAID12 corresponding to PsA disease activity states with good known groups validity. This indicates patients’ assessment reflects the disease process in PsA. One strength of our study is the use as external anchors to define disease activity based on both composite scores and the patient’s opinion. Proposed cutoff values for PsAID12 will be useful when defining treatment targets for PsA. Further validation is needed.
Reference: Pil Højgaard et al. Seminars in Arthritis and Rheumatism, 2017.
To cite this abstract in AMA style:
Gorlier C, Puyraimond-Zemmour D, Orbai AM, Coates LC, Kiltz U, Leung YY, Palominos P, Cañete JD, Scrivo R, Balanescu AR, Dernis E, Tälli S, Ruyssen-Witrand A, Soubrier M, Aydın SZ, Eder L, Gaydukova I, Lubrano E, Richette P, Husni ME, de Wit M, Smolen JS, Gossec L. Defining Cutoffs Corresponding to Low Levels of Disease Activity in Psoriatic Arthritis, Using the Patient-Reported Psoriatic Arthritis Impact of Disease Questionnaire (PsAID12). an Analysis of 436 Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/defining-cutoffs-corresponding-to-low-levels-of-disease-activity-in-psoriatic-arthritis-using-the-patient-reported-psoriatic-arthritis-impact-of-disease-questionnaire-psaid12-an-analysis-of-436-pa/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-cutoffs-corresponding-to-low-levels-of-disease-activity-in-psoriatic-arthritis-using-the-patient-reported-psoriatic-arthritis-impact-of-disease-questionnaire-psaid12-an-analysis-of-436-pa/