Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Electronic health record (EHR) data provide an inexpensive, information-rich tool to study rare diseases like antiphospholipid syndrome (APS). Many such studies rely on structured EHR data, e.g., diagnostic codes, laboratory testing, medication information. As a first step toward assessing the feasibility of using structured EHR data to study APS, we evaluated the presence and positivity of APS antibody testing in a diverse, US-based cohort using the All of Us Research Network Database (AOU).
Methods: Possible APS patients were identified by the presence of SNOMED-CT codes: 26843008 (Antiphospholipid syndrome) and 19267009 (Lupus anticoagulant disorder). Among possible APS patients, APS laboratory tests – anticardiolipin (aCL), anti-beta-2 glycoprotein I (aβ2GPI), and lupus anticoagulant (LA; confirmatory tests only) – were identified with a hand-curated list of LOINC and SNOMED-CT codes. Tests were identified as positive if they had a) a text result indicating positivity (e.g. positive, high, abnormal), b) a numeric result exceeding the normal reported range for that test, or c) a numeric result exceeding 40 if the test lacked a range, but included units of GPL, MPL, SGU, or SMU; negative tests were identified analogously. Tests without text or numeric results, ranges, or units, or tests with units other than those referenced above were classified as indeterminate. T-tests were two-sided with Bonferroni correction.
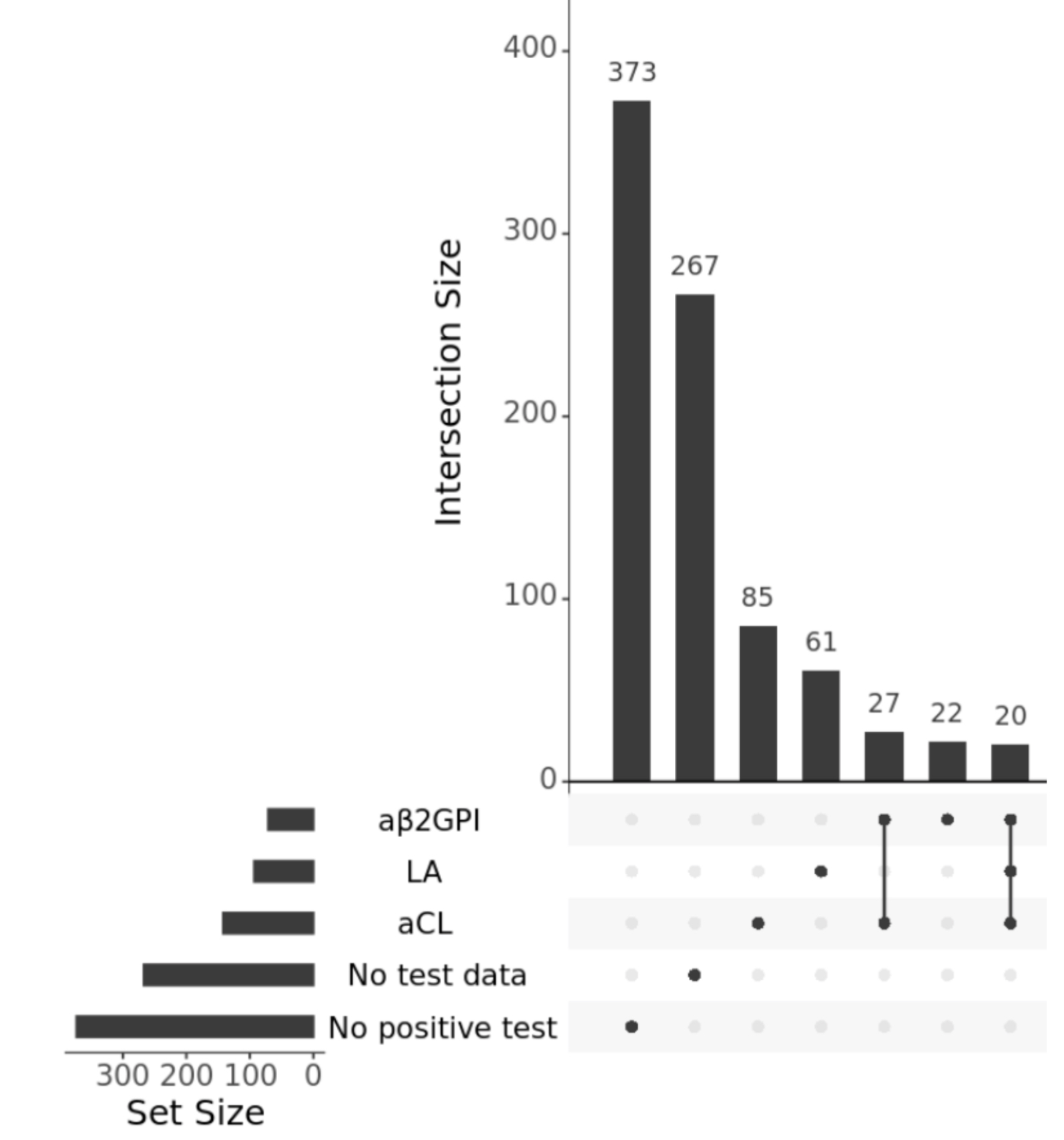
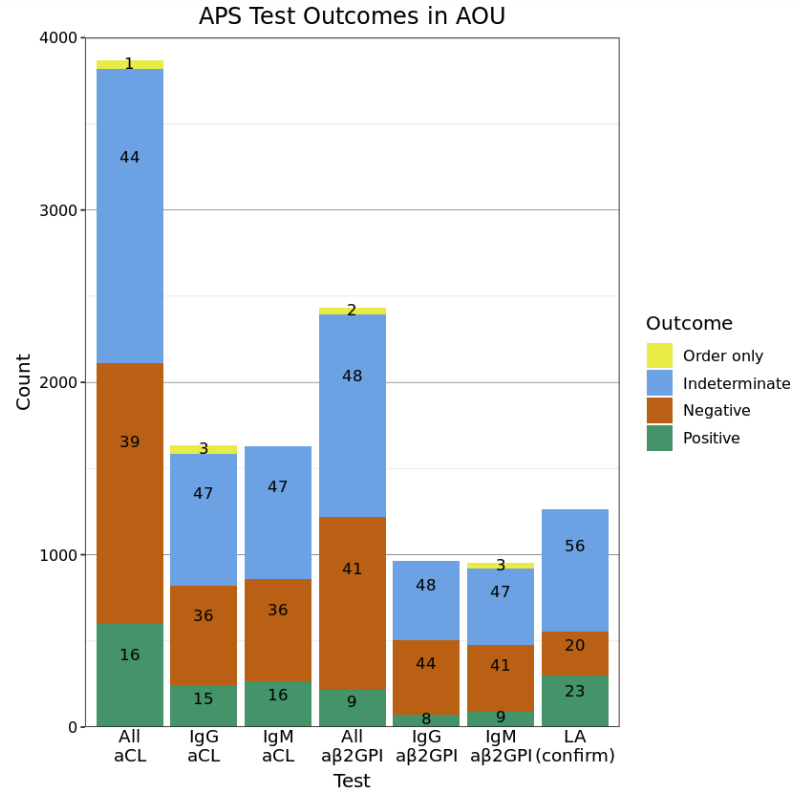
Results: Out of 372,082 total AOU patients, 883 (2.4 in 1,000) were identified as possible APS patients with demographic characteristics of 74% female, 55% White, and 17% Black, and a mean age of 55. Despite having testing information on 613 (70%) patients, only 434 (49%) had enough data to classify a test as positive or negative. Of these, 245 (28%) had any positive antiphospholipid test (Figure 1). Additionally, only 119 (13%) had two positive tests greater than 12 weeks apart, in accordance with APS classification criteria. Despite these low rates of positive tests, possible APS patients had on average 40 rows (i.e., orders and results) of APS test data compared to 1.7 rows among the non-APS patients. Percentages of positive tests were higher for LA (possibly because of its confirmatory nature) than aCL or aβ2GPI; percentages of indeterminate test results were high (44-56%) across all tests (Figure 2).
Conclusion: AOU offers one of the largest and most diverse datasets for studying the epidemiology and effects of APS. However, poor data quality can complicate the identification of a robust cohort of APS patients; only 1 in 8 patients with an APS diagnostic code in AOU met the laboratory criteria for APS, despite having an over-enrichment of APS test data compared to the whole database. This result is partially driven by half of all tests having an indeterminate result due to missing data. While improved standardization for testing and documentation could ameliorate data quality issues, creating a “digital phenotype” which combines many and different data types from the EHR will be needed to confidently identify likely APS patients for further study.
To cite this abstract in AMA style:
Balczewski E, Liang W, Ambati A, Zuo Y, Singh K, Knight J. Decoding Antiphospholipid Syndrome Laboratory Test Outcomes in a Large Multicenter Electronic Health Record Database [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/decoding-antiphospholipid-syndrome-laboratory-test-outcomes-in-a-large-multicenter-electronic-health-record-database/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/decoding-antiphospholipid-syndrome-laboratory-test-outcomes-in-a-large-multicenter-electronic-health-record-database/