Session Information
Date: Monday, November 13, 2023
Title: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Monoclonal antibody tumor necrosis factor alpha inhibitors, particularly infliximab and adalimumab, are the most commonly used biological agents in the treatment of Behçet’s syndrome (BS). Treatment response may show variability across organ manifestations of BS. We aimed to determine the frequency of de novo manifestations during adalimumab (ADA) treatment.
Methods: We conducted a chart review of 285 BS patients who received ADA in our Behçet Disease Research Center. Demographic data, reasons for initiating ADA, concurrent medications, previous treatments, and treatment outcomes were recorded. We defined de novo manifestations as new BS manifestations that had not manifested prior to the initiation of ADA treatment. For patients with vascular involvement, a new vascular event at another vessel was also considered as a de novo manifestation.
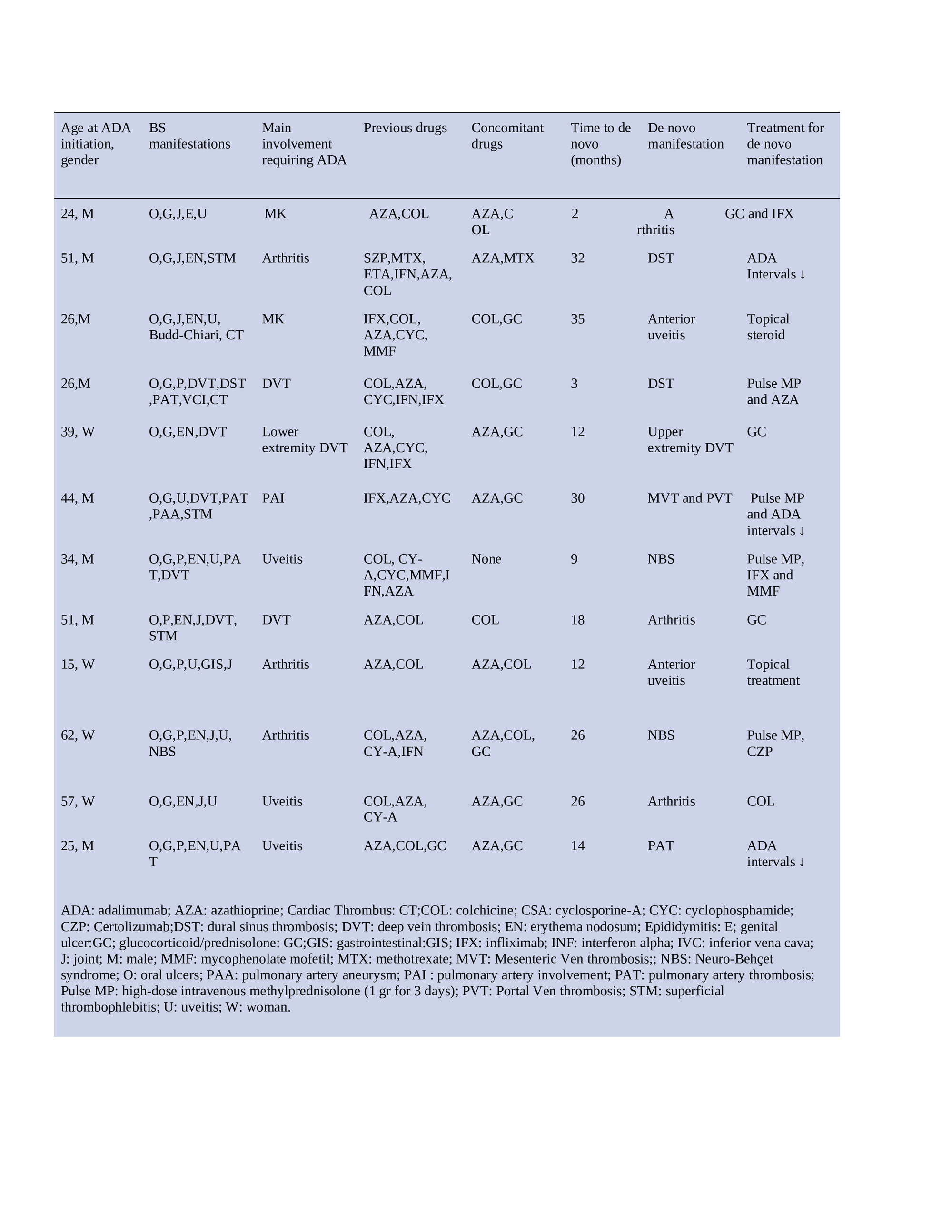
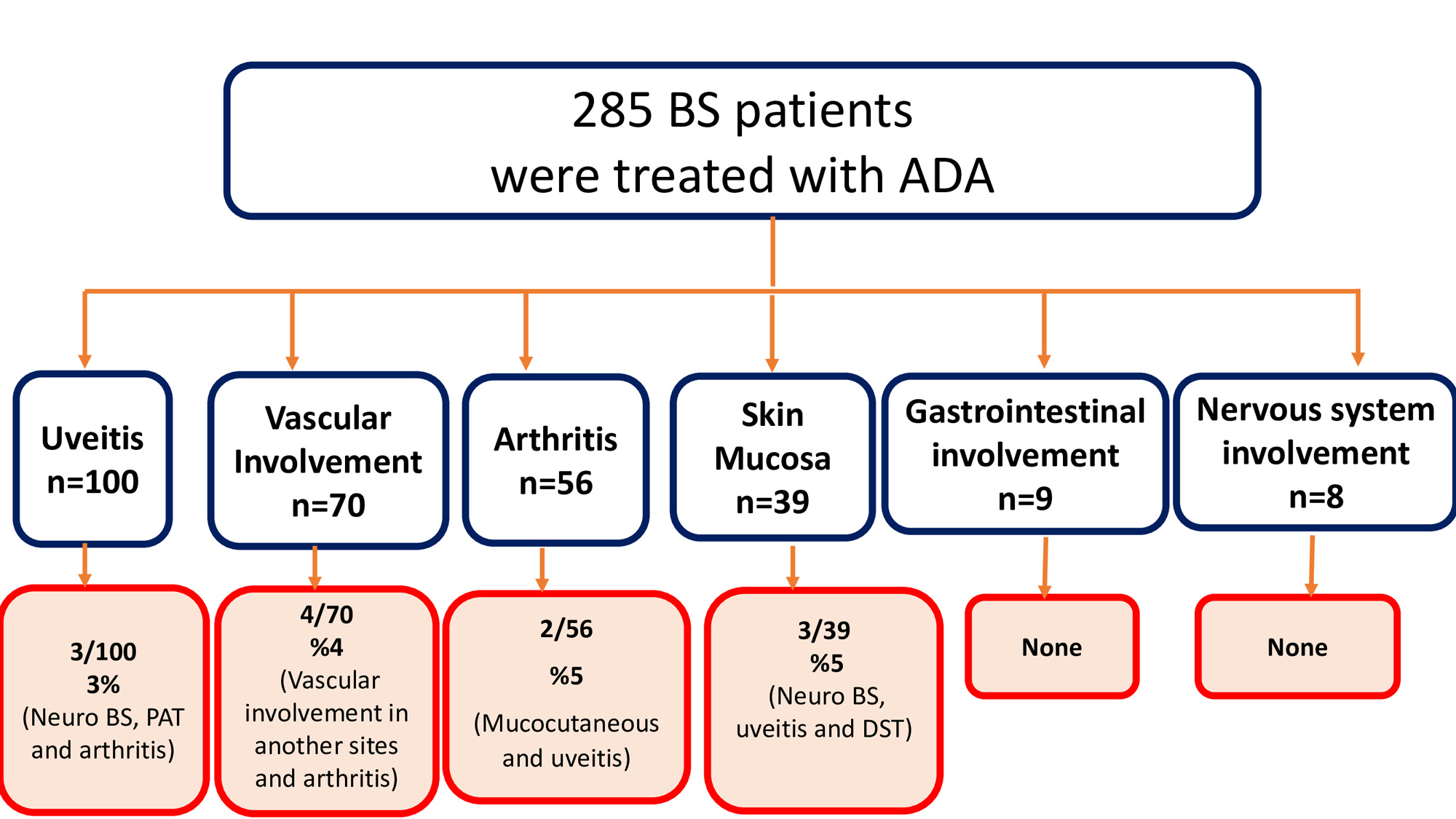
Results: The main reasons for ADA use among our 285 patients were uveitis in 100 patients (35%), vascular involvement in 70 (%25), arthritis in 56 (%20), mucocutaneous involvement in 39 (%14), parenchymal central nervous system involvement in 8 (%3), and gastrointestinal involvement in 9 (%3). Among these patients, 12 (4%) developed a de novo manifestation. De novo manifestations that occurred in 12 patients were vascular involvement in 5 patients, arthritis in 3, anterior uveitis in 2, and parenchymal central nervous system involvement in 2. The primary reasons for ADA treatment were vascular involvement in 3, arthritis in 3, uveitis in 3, and mucocutaneous involvement in 2 of these 12 patients. Among these 12 patients, 8 (61%) were using concomitant conventional immunosuppressive treatment at the time of occurrence of de novo manifestations.
Treatment with ADA was intensified in 3 patients by shortening the intervals to 1 week, along with the addition of high dose glucocorticoids in one patient. In the 3 patients, ADA was switched to another agent (infliximab in 2 patients, certolizumab in 1 patient). Only glucocorticoids were added in two patients, azathioprine along with high dose corticosteroids in one patient and colchicine in one patient. Two patients who had developed anterior uveitis were initiated topical treatment (Table).
Conclusion: De novo manifestations occurred in 4% of BS patients treated with adalimumab. Majority of these (75%) were major organ involvement, mainly vascular involement. None of the patients developed posterior uveitis, the 2 patients with uveitis developing during ADA had anterior uveitis that was controlled with topical agents.
12 patients
To cite this abstract in AMA style:
Esatoglu S, Sonmez O, Ucar D, Kaymaz E, Ozguler Y, Ugurlu S, Seyahi E, Melikoglu M, Fresko I, Hamuryudan V, Uygunoglu U, Kutlubay Z, Hatemi G. De Novo Manifestations During Adalimumab Treatment in Behçet Syndrome [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/de-novo-manifestations-during-adalimumab-treatment-in-behcet-syndrome/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/de-novo-manifestations-during-adalimumab-treatment-in-behcet-syndrome/