Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) clinical trials are challenged by high standard of care plus placebo (SOC+PBO) response rates.1 Previous post hoc analyses of the phase 2b RISE trial in SLE (NCT02804763) identified acute flare and normal complement as potential predictors of SOC+PBO response.2 This analysis aims to evaluate the treatment effect of dapirolizumab pegol (DZP; a polyethylene glycol-conjugated antigen-binding fragment lacking a functional Fc domain that inhibits CD40L) in subgroups of patients (pts) from the phase 2b RISE trial based on the previously identified potential predictors of SOC+PBO response.2,3
Methods: Post hoc analyses were performed on data from the phase 2b RISE trial, in which pts received PBO or DZP (6/24/45 mg/kg), alongside SOC, for 24 weeks (wks).3 Adults with moderately to severely active SLE, receiving stable doses of corticosteroids, antimalarial or immunosuppressant drugs were included in the trial.3 Pts from the trial were divided into subgroups based on previously identified potential predictors of SOC+PBO response.2 Disease course at screening was defined using British Isles Lupus Assessment Group (BILAG) 2004 item level scores as acute flare (worsening/new symptoms) or persistent (symptoms rated as the same) based on the past 4 wks compared with the 4 wks prior to those. Low C3/C4 was defined as below the lower limit of normal at screening. Outcomes to Wk 24 were evaluated in subgroups and compared with the overall population. Outcomes assessed were BILAG-based Composite Lupus Assessment (BICLA) response, SLE Responder Index-4 (SRI-4) response, and change from baseline in Physician’s Global Assessment (PGA).
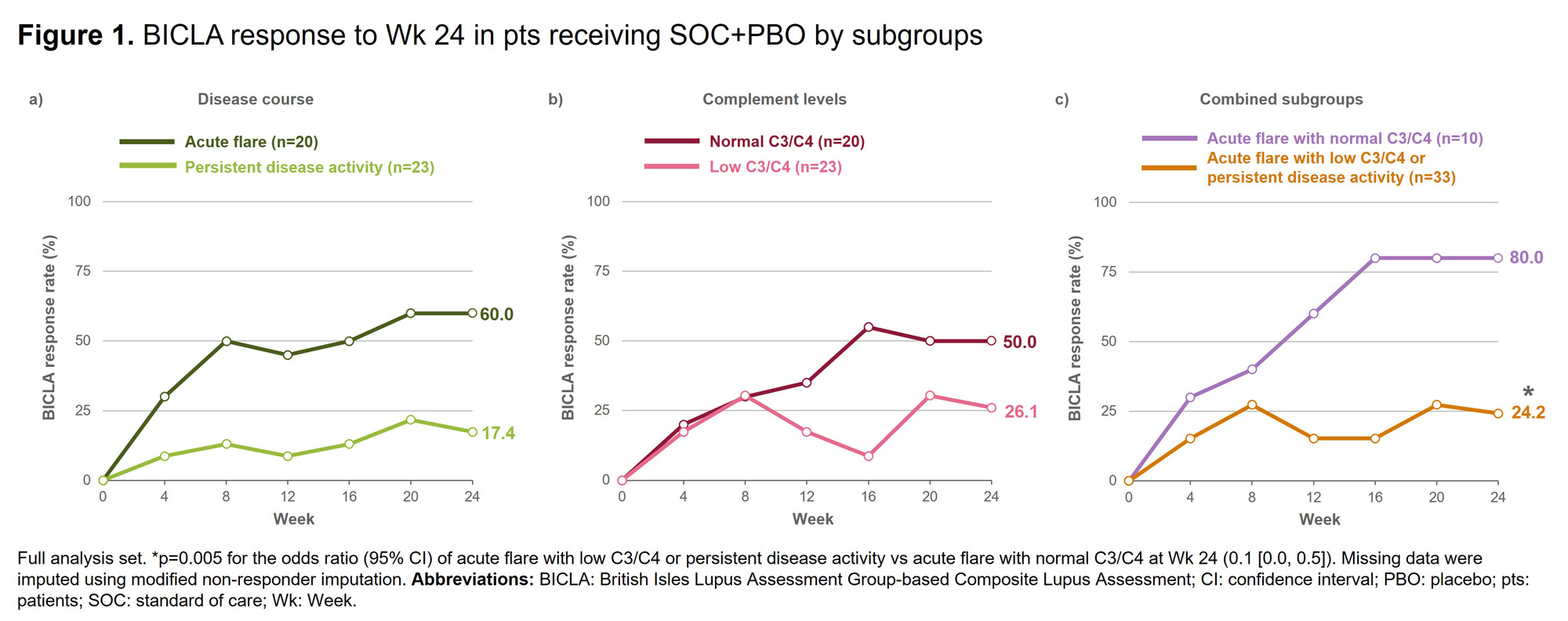
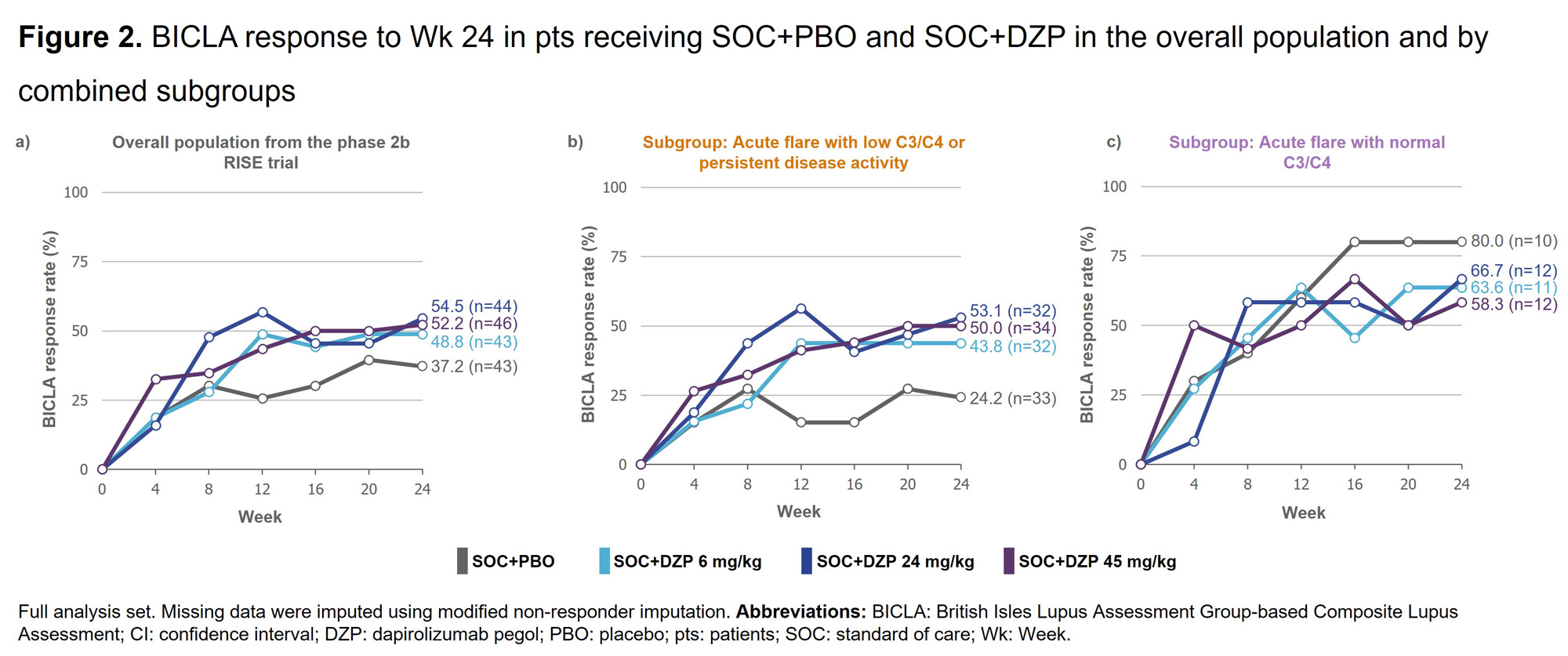
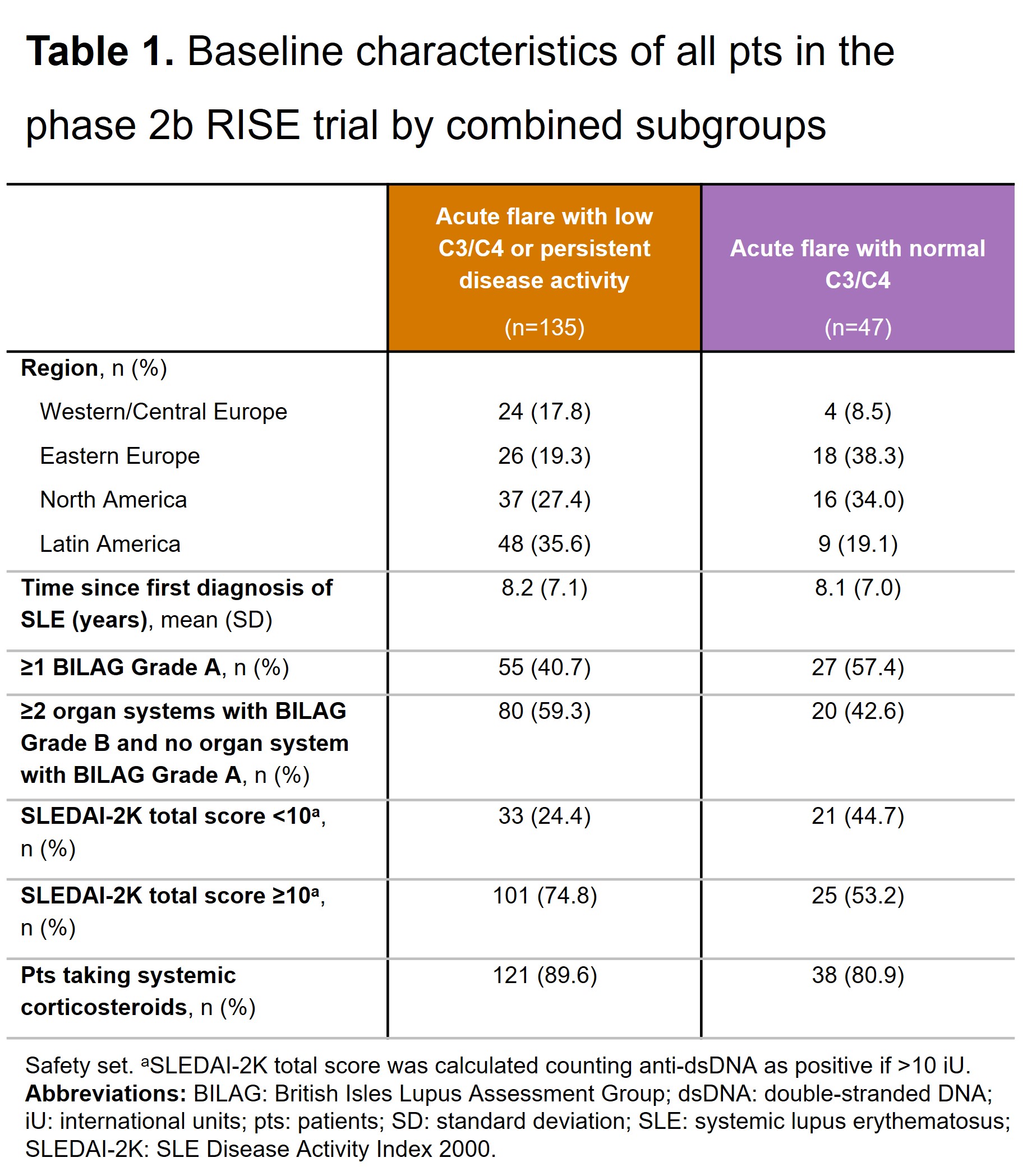
Results: A higher proportion of pts receiving SOC+PBO with acute flare (vs persistent disease activity) and with normal C3/C4 (vs low C3/C4) achieved BICLA response at Wk 24 (Figures 1a&1b). When the subgroups were combined, a significantly higher proportion of pts receiving SOC+PBO with acute flare and normal C3/C4 achieved BICLA response at Wk 24 compared with pts receiving SOC+PBO with either acute flare with low C3/C4 or persistent disease activity (Figure 1c). BICLA responses to Wk 24 in the combined subgroups for pts receiving SOC+PBO and SOC+DZP are presented in Figure 2b&2c, alongside BICLA response in the overall population from the RISE trial in Figure 2a. Similar patterns were seen for SRI-4 response and change from baseline in PGA in the combined subgroups (data not shown). Baseline characteristics in the combined subgroups are presented in Table 1.
Conclusion: Albeit in a limited sample size, pts with acute flare and normal complement were more likely to achieve response to SOC+PBO than pts with acute flare and low complement or persistent disease activity. These data suggest that SLE trial design may need to consider baseline clinical and serologic activity patterns to adequately assess treatment efficacy. References:1. Durcan L. Lancet. 2023;S0140-6736(23)00342-2. 2. Stach C. Presented at LUPUS & KCR 2023, Abstract 2023-0801, ePoster LP-198. 3. Furie RA. Rheumatology (Oxford). 2021;60:5397–407.
To cite this abstract in AMA style:
Askanase A, Stach C, Brittain C, Stojan G, Furie R. Dapirolizumab Pegol Efficacy by Subgroups in Patients with SLE: A Post Hoc Analysis of Phase 2b Clinical Trial Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dapirolizumab-pegol-efficacy-by-subgroups-in-patients-with-sle-a-post-hoc-analysis-of-phase-2b-clinical-trial-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dapirolizumab-pegol-efficacy-by-subgroups-in-patients-with-sle-a-post-hoc-analysis-of-phase-2b-clinical-trial-data/