Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Damage Index in APS (DIAPS) is a scoring system developed to assess long-term damage in thrombotic primary antiphospholipid syndrome (PAPS), which also correlates with impaired quality of life (EuroQoL) in Latin Americans. DIAPS is not validated in aPL-positive patients without thrombosis. Our primary objective was to quantify damage accrual measured by DIAPS in aPL-positive patients with or without a history of thrombosis in an international cohort. Secondly, we aimed to identify clinical and laboratory characteristics associated with damage in aPL-positive patients.
Methods: In this cross-sectional study, we analyzed the baseline damage, measured by DIAPS, in APS ACTION Registry patients. The inclusion criteria were positive aPL according to Updated Sapporo Classification Criteria tested within one year prior to enrollment. We excluded patients with other autoimmune diseases. We analyzed the demographic, clinical, and laboratory characteristics of patients based on two subgroups: (1) thrombotic APS patients with high (DIAPS ≥3) versus low damage (DIAPS < 3); and (2) non-thrombotic aPL-positive patients with damage (DIAPS >0) versus without damage (DIAPS=0). Chi-square, Fisher’s exact test, Mann-Whitney U and Student t test were used when applicable. In the multivariate analysis, our model included age, gender, race and variables with p< 0.10 in the univariate analysis.
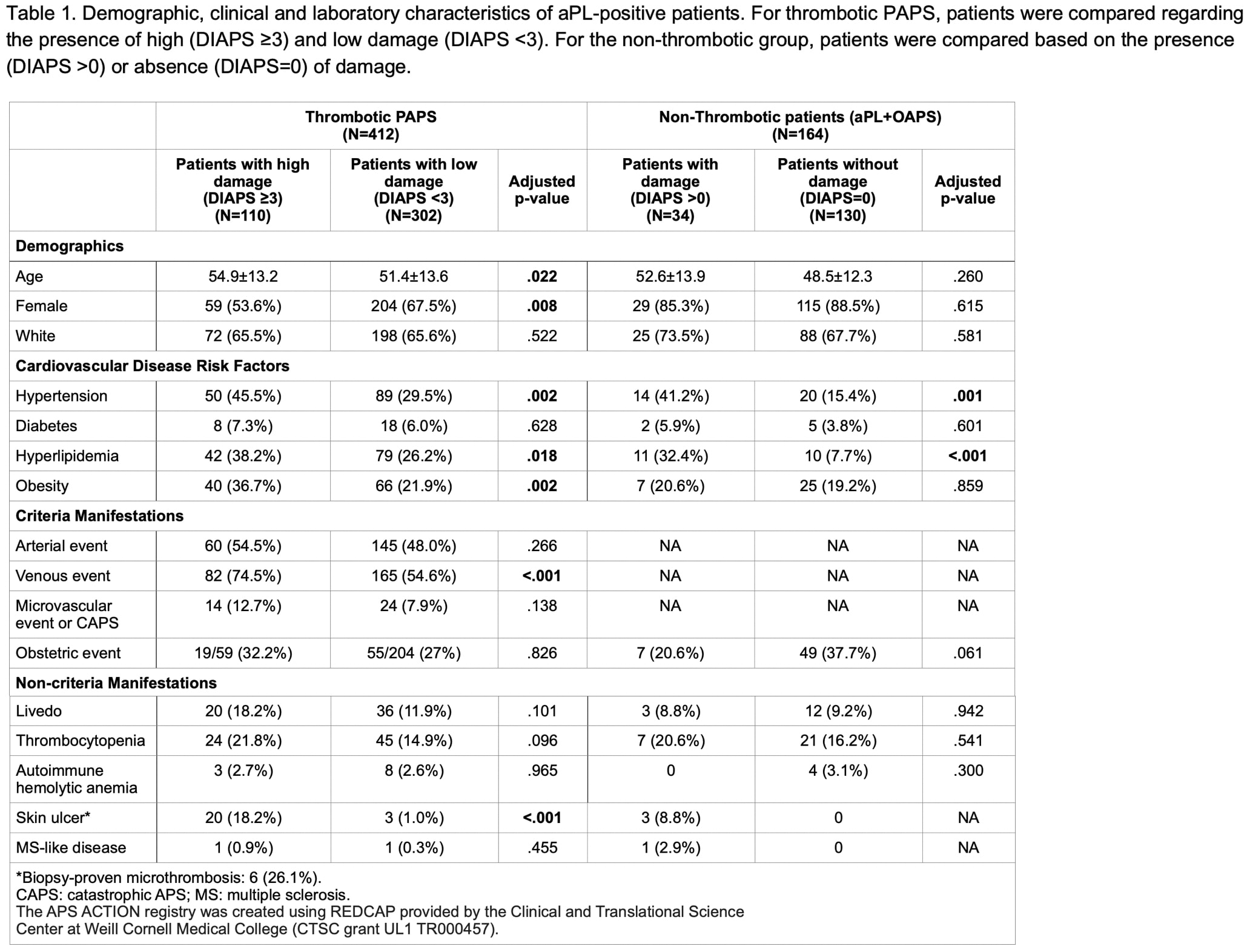
Results: Of the 826 aPL-positive patients included in the registry as of April 2020, 576 with no other systemic autoimmune diseases were included in the analysis (412 thrombotic and 164 non-thrombotic [108 aPL only and 56 obstetric]). Baseline demographic, clinical and laboratory characteristics are summarized in Table 1. For the thrombotic group, the most frequent domains contributing to damage were peripheral vascular (n=260, 63% – mainly deep vein thrombosis), neuropsychiatric (n=107, 30% – mainly ischemic stroke with sequelae) and cardiovascular (n=57, 14% – mainly heart valve disease). Older age, male gender, hypertension, hyperlipidemia and obesity were associated with high damage (Table 1). In the multivariate analysis, male gender (OR 1.73, 95%CI 1.10-2.71, p=.018) and hypertension (OR 1.90, 95%CI 1.21-2.99, p=.006) were independently correlated with high damage. For the non-thrombotic group, the most frequent domains contributing to damage were neuropsychiatric (n=25, 15% – mainly cognitive impairment) and cardiovascular (n=13, 8% – mainly heart valve disease). Hypertension and hyperlipidemia were independently associated with damage in the multivariate analysis (OR 2.72, 95%CI 1.09-6.80, p=.032 and OR 4.48, 95%CI 1.62-12.29, p=.004, respectively). There was no correlation between aPL profile (triple vs double vs single aPL) and damage in either group.
Conclusion: DIAPS was able to discriminate damage in a large multicenter cohort of aPL-positive patients. Traditional cardiovascular risk factors, namely older age, male gender, hypertension, hyperlipidemia and obesity, correlate with higher damage in thrombotic primary APS patients. Hypertension and hyperlipidemia also correlate with damage in aPL-positive patients without a history of thrombosis.
To cite this abstract in AMA style:
Balbi G, Ahmadzadeh Y, Tektonidou M, Pengo V, Sciascia S, Ugarte A, Belmont H, Gerosa M, Fortin P, lopez-pedrera C, Ji L, Atsumi T, Cohen H, de Jesus G, Branch D, Nalli C, Kello N, Petri M, Rodriguez-Almaraz E, Barilaro G, Knight J, Artim-Esen B, Willis R, Bertolaccini M, Roubey R, Erkan D, De Andrade D, APS ACTION o. Damage Accrual Measured by DIAPS in Antiphospholipid Antibody (aPL)-positive Patients: Results from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/damage-accrual-measured-by-diaps-in-antiphospholipid-antibody-apl-positive-patients-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-cli/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/damage-accrual-measured-by-diaps-in-antiphospholipid-antibody-apl-positive-patients-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-cli/