Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Vasculitis is a group of rare, multisystem diseases which may involve the skin. Characterization of the frequency, type, and significance of cutaneous manifestations in various vasculitides is incomplete.
Methods: This study analyzed cross-sectional data collected through the Diagnostic and Classification Criteria in Vasculitis (DCVAS) (NCT01066208) study, a large, multinational, collaborative effort involving 136 clinical sites in 32 countries from 2011 to 2017, collecting comprehensive clinical data on patients with several forms of vasculitis from disease onset to 6 months after initial presentation. Summary statistics were used to describe cutaneous manifestations of each included type of vasculitis, and logistic regression analysis was used to calculate the odds ratio for severe systemic manifestations of vasculitis based on presence of skin findings.
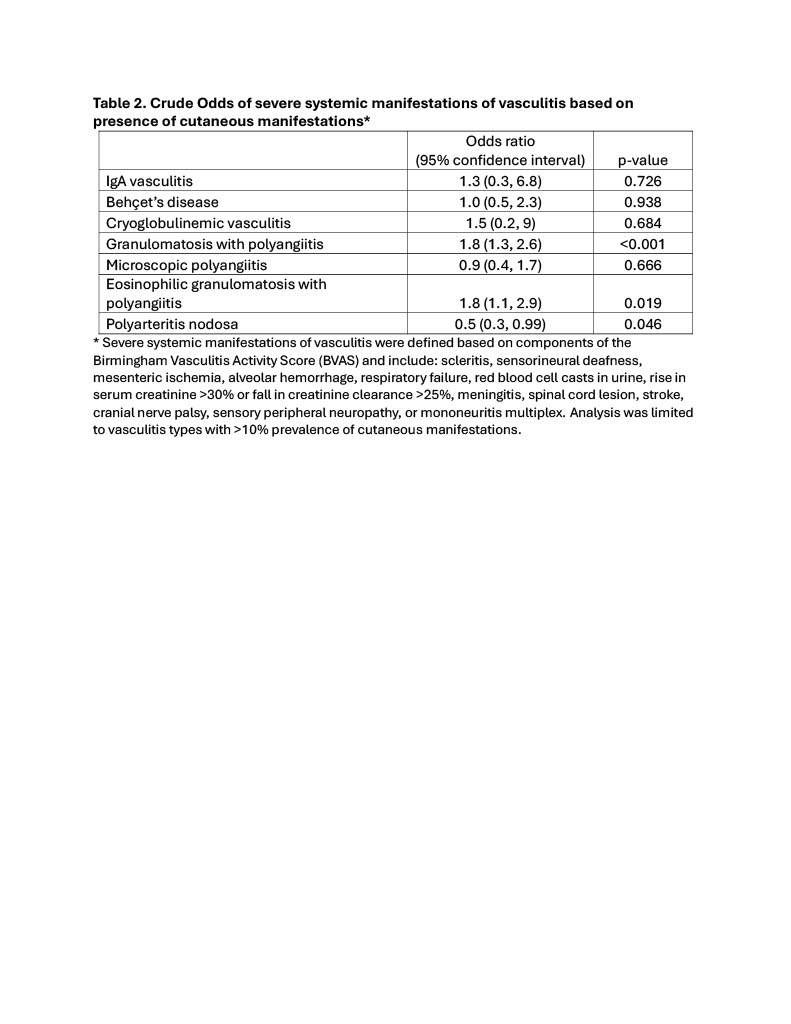
Results: Data from 4,468 adults diagnosed with vasculitis were analyzed, including 270 with IgA vasculitis (IgAV), 201 with Behçet’s disease (BD), 57 with cryoglobulinemic vasculitis (CV), 1,023 with granulomatosis with polyangiitis (GPA), 505 with microscopic polyangiitis (MPA), 382 with eosinophilic granulomatosis with polyangiitis (EGPA), 194 with polyarteritis nodosa (PAN), 1,206 with giant cell arteritis (GCA), and 630 with Takayasu arteritis (TAK). Participants in the DCVAS study were from Europe (59%), North America (21%), Asia (17%), Oceania (2%), and Africa (1%). Cutaneous manifestations were common at the time of presentation for all types of vasculitis except large-vessel vasculitis (GCA and TAK), ranging from 20% of patients with MPA to 97% of patients with IgAV (Table 1). The frequency of specific cutaneous manifestations encountered in each type of vasculitis varied considerably, although petechiae/purpura was the most common skin finding in all types of small-vessel vasculitis (IgAV, CV, GPA, MPA, and EGPA). Skin biopsy was utilized to varying degrees, performed in 22-82% of those with small- or medium-vessel vasculitis. When performed, skin biopsy was diagnostic in the majority of cases, including 95% (205/216) of IgAV, 73% (16/22) of CV, 73% (67/92) of GPA, 73% (16/22) of MPA, 67% (49/73) of EGPA, and 70% (52/74) of PAN. Skin biopsy was less often useful for diagnosis of BD (35%; 7/20), GCA (0%; 0/1), and TAK (33%; 2/6). Univariable logistic regression demonstrated that patients with cutaneous manifestations had significantly greater odds of severe systemic manifestations of GPA (OR 1.8, 95% CI 1.3, 2.6) and EGPA (OR 1.8, 95% CI 1.1, 2.9); those with cutaneous manifestations had lower odds of severe systemic manifestations of PAN (OR 0.5, 95% CI 0.3, 0.99) (Table 2).
Conclusion: These findings characterize the cutaneous manifestations of vasculitis at the time of diagnosis and highlight the potential diagnostic and prognostic importance of the skin in these multisystem diseases. For patients presenting with small- and medium-vessel vasculitis, early recognition of cutaneous manifestations, combined with the high diagnostic yield of skin biopsy, may help accelerate accurate diagnosis, preclude unnecessary testing, and shorten time to initiation of disease-specific therapy.
.jpg) Severe systemic manifestations of vasculitis were defined based on components of the Birmingham Vasculitis Activity Score (BVAS) and include: scleritis, sensorineural deafness, mesenteric ischemia, alveolar hemorrhage, respiratory failure, red blood cell casts in urine, rise in serum creatinine >30% or fall in creatinine clearance >25%, meningitis, spinal cord lesion, stroke, cranial nerve palsy, sensory peripheral neuropathy, or mononeuritis multiplex. Analysis was limited to vasculitis types with >10% prevalence of cutaneous manifestations.
Severe systemic manifestations of vasculitis were defined based on components of the Birmingham Vasculitis Activity Score (BVAS) and include: scleritis, sensorineural deafness, mesenteric ischemia, alveolar hemorrhage, respiratory failure, red blood cell casts in urine, rise in serum creatinine >30% or fall in creatinine clearance >25%, meningitis, spinal cord lesion, stroke, cranial nerve palsy, sensory peripheral neuropathy, or mononeuritis multiplex. Analysis was limited to vasculitis types with >10% prevalence of cutaneous manifestations.
To cite this abstract in AMA style:
Micheletti R, Song W, Chu B, Allen-Taylor L, Gelfand J, Grayson P, Ponte C, Robson J, Suppiah R, Luqmani R, Watts R, Merkel P. Cutaneous manifestations of vasculitis: A cross-sectional analysis from an international cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cutaneous-manifestations-of-vasculitis-a-cross-sectional-analysis-from-an-international-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cutaneous-manifestations-of-vasculitis-a-cross-sectional-analysis-from-an-international-cohort/