Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 inhibitor approved in multiple countries for the treatment of adults with plaque psoriasis. A 48-week, double-blind phase 2 trial (NCT03252587) in patients with SLE showed that deucravacitinib demonstrated greater efficacy compared with placebo across multiple endpoints, including the primary endpoint of Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) at week 32 and the secondary endpoint of ≥50% reduction in CLASI activity (CLASI-50) for patients with moderate to severe skin involvement at baseline (CLASI score ≥10).1 Here, we explored whether patients could sustain CLASI-50 over time and achieve 100% reduction in CLASI activity. CLASI-50 was also evaluated by SLE cutaneous manifestation subtype.
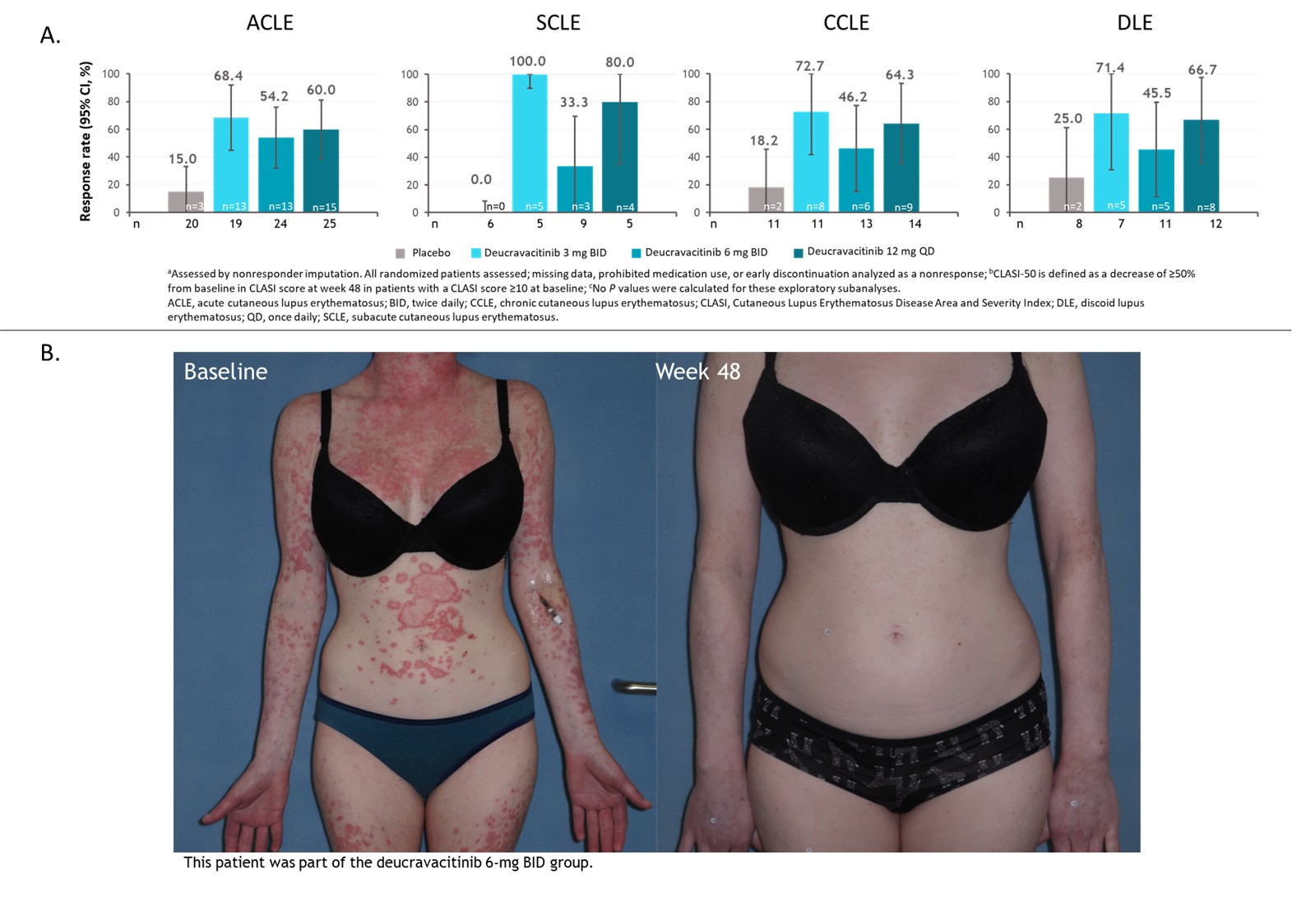
Methods: Patients with active SLE and a baseline CLASI score ≥10 who received placebo (n=24), deucravacitinib 3 mg twice daily (BID) (n=23), 6 mg BID (n=25), or 12 mg once daily (n=29) were included in these analyses. Exploratory outcomes were the proportion of patients that sustained CLASI-50 for the 5 consecutive visits from weeks 32 through 48, achieved 100% reduction in CLASI activity at week 48, and achieved CLASI-50 by SLE cutaneous manifestation subtype. For the subtype analysis, patients were classified as acute, subacute, chronic, or discoid at the discretion of investigators using SLICC classification criteria at screening without biopsy confirmation. Patients could be in ≥1 subcategory based on classification criteria; all patients classified as discoid were included in the chronic subgroup. All analyses were descriptive.
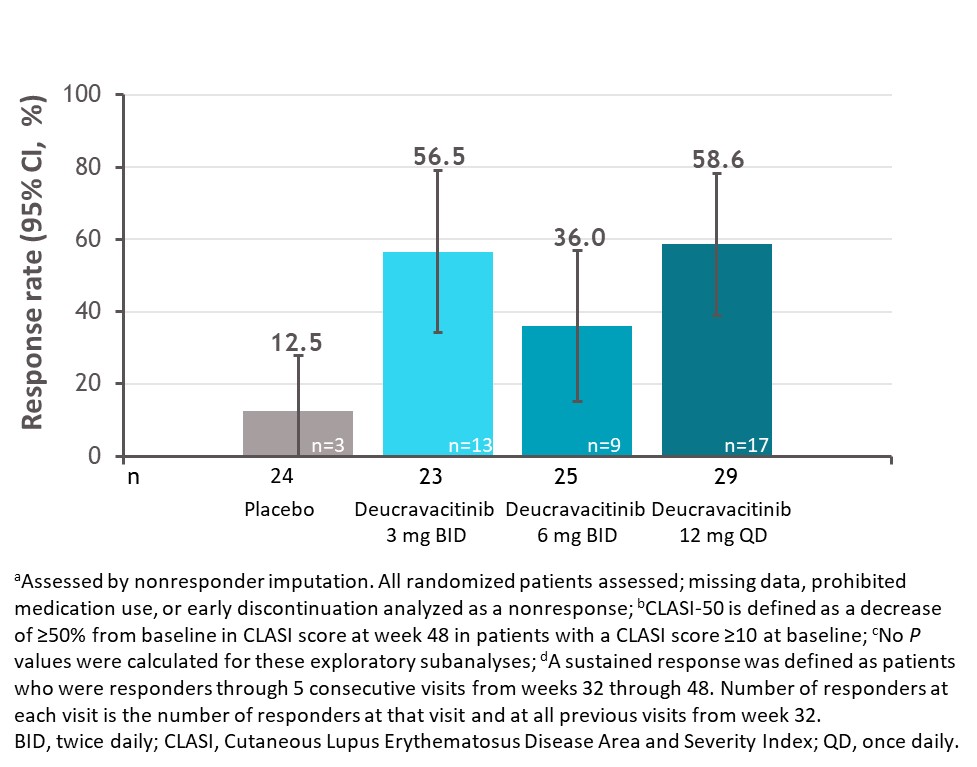
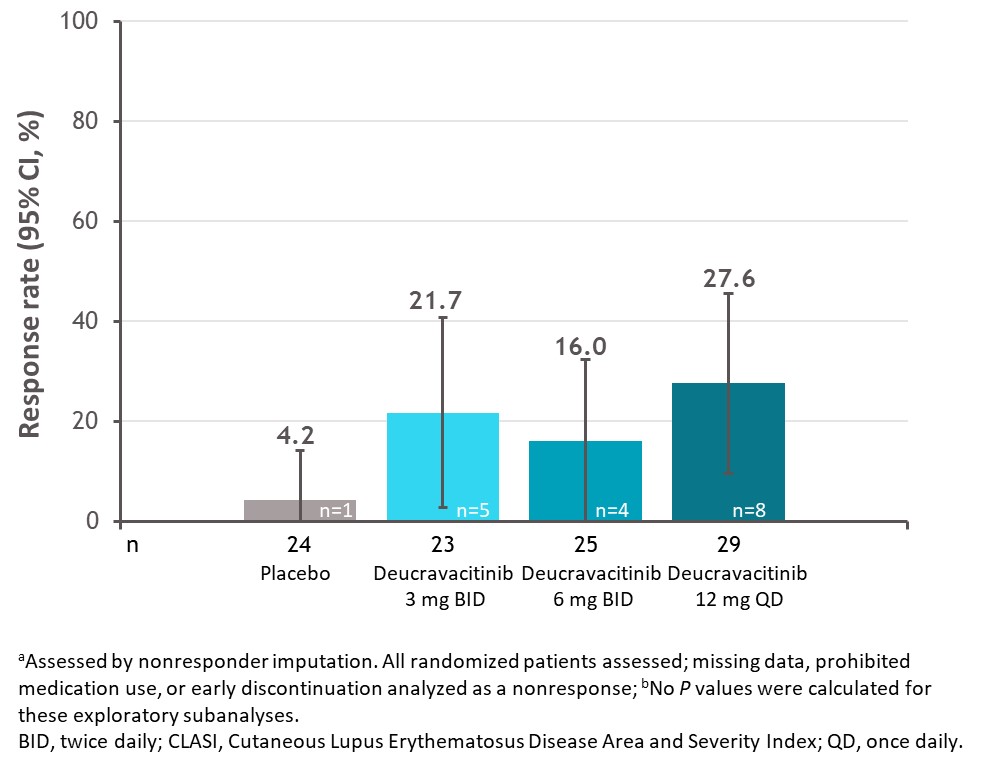
Results: Among patients with a baseline CLASI score ≥10, a numerically higher proportion of patients treated with deucravacitinib had a sustained response for the 5 consecutive visits between weeks 32 and 48 compared with placebo (Figure 1). Furthermore, a higher proportion of these patients treated with deucravacitinib achieved 100% reduction in CLASI activity at week 48 (Figure 2). Analysis by cutaneous manifestation suggested that a numerically higher proportion of patients treated with deucravacitinib achieved CLASI-50 at week 48 compared with placebo in all subtypes assessed (Figure 3).
Conclusion: Among patients with moderate to severe skin involvement at baseline, more patients treated with deucravacitinib were able to achieve improvements in skin overall, and a greater proportion of patients were able to sustain a CLASI-50 response from weeks 32 through 48 compared with placebo. CLASI-50 achievement was more frequent among patients treated with deucravacitinib regardless of cutaneous subtype.
Reference:
1. Morand E, et al. Arthritis Rheumatol 2023;75:242–252.
To cite this abstract in AMA style:
Arriens C, van Vollenhoven R, Gottlieb A, Hobar C, Pomponi S, Koti R, Wegman T, Werth V. Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) Achievement and Sustained Response with Deucravacitinib, an Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in a Phase 2 Trial in SLE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cutaneous-lupus-erythematosus-disease-area-and-severity-index-clasi-achievement-and-sustained-response-with-deucravacitinib-an-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-a-phase-2/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cutaneous-lupus-erythematosus-disease-area-and-severity-index-clasi-achievement-and-sustained-response-with-deucravacitinib-an-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-a-phase-2/