Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite significant improvement in its management, macrophage activation syndrome (MAS) treatment is still not standardized, due to lack of robust evidence and differences in access to medications, especially for MAS related to rheumatologic conditions other than systemic juvenile idiopathic arthritis (sJIA). We aimed to capture the current treatment of MAS worldwide and to evaluate major unmet needs
Methods: In context of the METAPHOR project, a PReS/PRINTO initiative to optimize treatment in sJIA and MAS,
a survey on MAS treatment was developed. Topics addressed were based on a systematic literature review, and selected by a panel of 22 experts, including 1 patient representative and 2 pediatric hematologists. The survey consisted of: a) demographic section, including country of practice; b) clinical section, investigating real-life approaches to MAS; c) patient focused section, developed with patient representatives and exploring major unmet needs. Physicians part of the PReS/PRINTO network and of the Histiocyte Society were invited to anonymously complete the web-survey from Oct 5th to Dec 15th2023
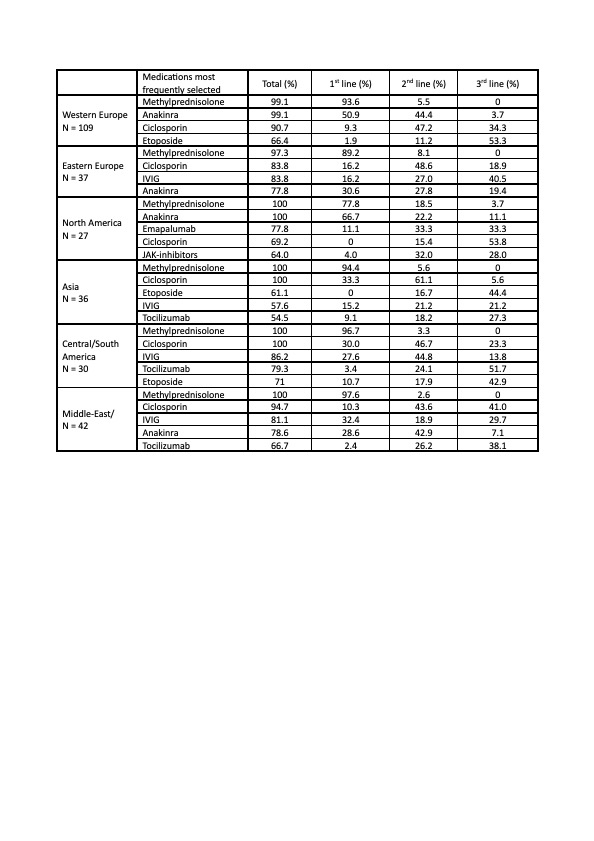
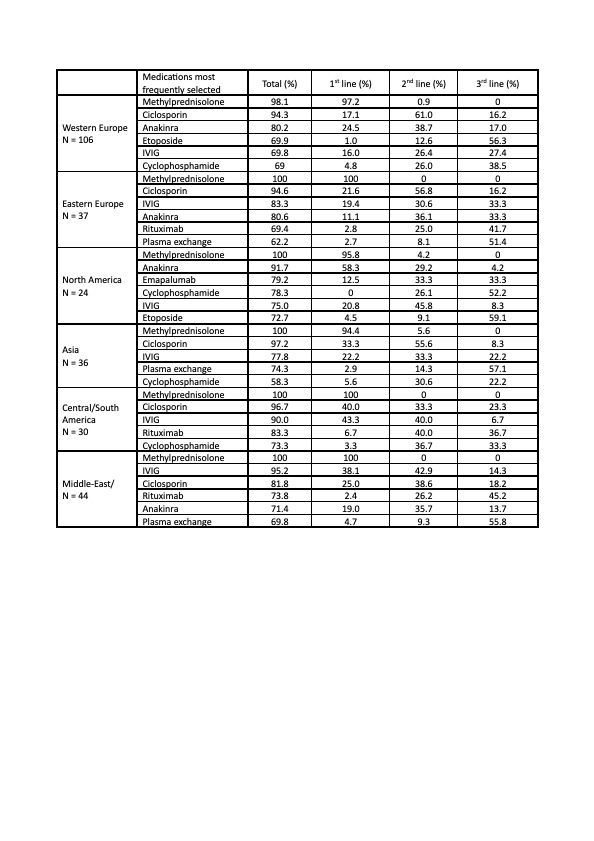
Results: A total of 287 replies from 64 countries worldwide were collected. Respondents were mostly pediatric rheumatologists (90%), while 10% were pediatric hematologists. Methylprednisolone (MPN) pulses were the commonest glucocorticoid (GC) in all subtypes of MAS. In addition to GC, ciclosporin (CsA) and anakinra were the cornerstones of treatment in sJIA-MAS, used by 91% and 74% of physicians, respectively. Anakinra was the most selected agent beside GC as 1st line, and only 2% of respondents indicated not to consider the use anakinra in sJIA-MAS. However, access to anakinra is a major gap across countries: almost all physicians would use it in North America and Western Europe, but it is still unavailable for more than 65% and 70% of physicians in Asia and South America (Table 1). Systemic erythematosus lupus (SLE)-associated MAS represents the condition with the highest heterogeneity of therapeutic approaches across centres and countries. Besides MPN, which was largely the 1st line choice worldwide (98%), 58% of physicians would use anakinra as 1st line in North America and 63% as 1st/2nd line in Western Europe, while CsA and immunoglobulin would be the medications of choice in all the other countries (Table 2). Etoposide was globally the agent most frequently selected as 3rd line/rescue, followed by JAK-inhibitors, mainly ruxolitinib. Emapalumab was potentially chosen as 2nd/3rd line treatment across all subtypes of MAS; however, its access is still drastically limited in all countries, except North America and partially Western Europe
Conclusion: A wide heterogeneity in the approach to MAS still exists, with relevant discrepancies in its management worldwide. An international effort is needed to optimize therapeutic options, reduce gaps in access to medications and harmonize treatment
To cite this abstract in AMA style:
Minoia F, Baldo F, Erkens R, Rogani G, Bracaglia C, Foell D, Gattorno M, Jelusic M, Anton J, Brogan P, Canna S, Chandrakasan S, Cron R, De Benedetti F, Grom A, Heshin Bekenstein M, Horne A, Khubchandani R, Mizuta M, Ozen S, QUARTIER P, Ravelli A, Shimizu M, schulert g, Scott C, Sinha R, Ruperto N, Swart J, Vastert S. Current Treatment of Macrophage Activation Syndrome Worldwide: The METAPHOR Project, a PReS/PRINTO Real-life International Survey [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/current-treatment-of-macrophage-activation-syndrome-worldwide-the-metaphor-project-a-pres-printo-real-life-international-survey/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/current-treatment-of-macrophage-activation-syndrome-worldwide-the-metaphor-project-a-pres-printo-real-life-international-survey/