Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Following the Coronavirus Disease-19 (COVID-19) pandemic outbreaks, the hyperinflammatory condition termed Multisystem Inflammatory Syndrome in Children (MIS-C) has become a healthcare issue worldwide. Since December 2020 the mRNA vaccine against SARS-CoV-2 has become available with a good safety profile. However, evidence regarding safety and vaccination strategies in children with previous MIS-C is still lacking. The aim of our study was to investigate the current approach in different international centres to COVID-19 and other vaccinations among children with previous MIS-C
Methods: Physician all around the globe who take care of patients with MIS-C were invited to anonymously complete a 15-question web-based survey. The survey was open from October 6th to December 31st 2021. Centre, country and specialty of the participants were collected. Participants were asked to describe their current vaccination strategy for MIS-C patients and to provide the most important variables affecting their decision-making process
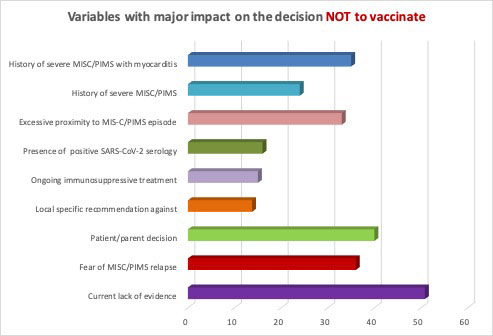
Results: A total of 290 replies from 237 centres in 61 countries were collected. Most respondents (86%) were pediatric rheumatologists. The anti-COVID-19 vaccine was available in 85% of the countries covered by the survey. Sixty-seven centres (28%) from 22 countries had already vaccinated MIS-C patients (< 5 patients: 52%; 5-10 patients: 29%; > 10 patients: 20%), without adverse reactions in most cases (89%). Six centres reported complications after the anti-COVID-19 vaccine: 2 not specified, 3 mild symptoms (fever, sore arm) and only 1 centre reported a MIS-C like reaction. MIS-C patients re-infected by SARS-CoV-2 were seen in 15% of centres and 2 of them reported a MIS-C flair. Most centres (84%) were in favour of vaccinating MIS-C patients against SARS-CoV-2, waiting 3-6 months (40%), 6-12 months (52%) or > 12 months (8%) after a MIS-C episode. The variable with the greatest impact on the decision not to vaccinate MIS-C patients was the current lack of evidence (51%), followed by patient/parent decision, fear of MIS-C relapse and history of severe MIS-C with myocarditis (Figure 1). The most relevant parameters in the vaccination strategy were time from MIS-C episode (78%), ongoing immunosuppressive treatment (35%), SARS-CoV-2 serologic status (32%) and MIS-C features (31%). Almost all centres favoured continuing regular vaccination with non-live (99%) and live (93%) vaccines, waiting 3-6 months and 6-12 months, respectively
Conclusion: When vaccinating MIS-C patients against SARS-CoV-2, the experience reported by the international pediatric rheumatology community to date is overall reassuring. However, lack of evidence still affects the vaccination strategy of many centres worldwide. Large, prospective studies are needed to properly evaluate the safety of anti-COVID-19 vaccination among MIS-C patients
To cite this abstract in AMA style:
Minoia F, Lucioni F, Heshin-Bekenstein M, Vastert S, Kessel C, Uziel Y, Lamot L, Ruperto N, Gattorno M, Bracaglia C, Toplak N. Current Approach to COVID-19 and Other Vaccinations in Children with Previous Multisystem Inflammatory Syndrome (MIS-C): An International Survey [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/current-approach-to-covid-19-and-other-vaccinations-in-children-with-previous-multisystem-inflammatory-syndrome-mis-c-an-international-survey/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/current-approach-to-covid-19-and-other-vaccinations-in-children-with-previous-multisystem-inflammatory-syndrome-mis-c-an-international-survey/