Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Functional Status Assessment Measures (FSAMs) are important outcome measures in Rheumatoid Arthritis (RA) as poor function is a predictor for mortality and associated with lower quality of life and work disability. FSAMs inform assessment and treatment as part of guideline-based care. The Health Assessment Questionnaire (HAQ) and its derivatives are standardized and validated FSAMs commonly used in RA. More recently the Patient-Reported Outcomes Measurement Information System (PROMIS) has been developed and includes FSAMs. The HAQ and PROMIS measures were developed and validated in English, but have been translated and culturally adapted for use in other countries. Our objective was to conduct a systematic review of the cross-cultural validity of FSAMs for RA including HAQ and HAQ-derived measures as well as PROMIS measures.
Methods: Four electronic medical databases (MEDLINE, EMBASE, Cochrane Library and CINHAL) were searched in accordance with a published strategy from the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) group. The references of each included article were then manually searched for additional relevant studies. Included studies were evaluated using the COSMIN tool for cross-cultural validity and were scored as excellent, good, fair or poor.
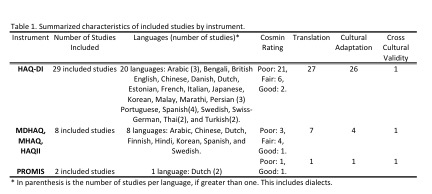
Results: Of 58 papers identified, by our search strategy and 3 identified by manual search, 39 were included: 29 described the translation, cultural adaptation or cross-cultural validity of the HAQ-DI, 8 other HAQ derivatives, and 2 the PROMIS measures, representing 22 languages (Table). Of the 39 papers reviewed, 35 described translation, 31 described cultural adaptation, and only 3, examined cross-cultural validity of translated versions. There was generally poor adherence to proposed guidelines for cross-cultural adaptations of measures. No studies were rated as excellent by the COSMIN tool, only 4 studies were rated as good, 10 were rated fair, and 25 were rated as poor. Two studies that looked at cross-cultural validity noted differential item functioning (DIF) between Dutch and US populations for the HAQ-II and PROMIS measures and a third study found DIF between Turkish and UK populations on the HAQ.
Conclusion: FSAMs have been widely used both in their validated English form and in many translated forms. This review highlights a paucity of data on the cross-cultural validity of FSAMs and the mostly poor or fair quality methods by which they were translated and adapted. This needs to be considered when using these measures for multinational clinical trials and for day-to-day use in practice.
To cite this abstract in AMA style:
Kulhawy-Wibe S, Zell J, Michaud K, Yazdany J, Ehrlich-Jones LS, Thorne C, Everix D, Barber C. Cross-Cultural Validity of Functional Status Assessment Measures for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/cross-cultural-validity-of-functional-status-assessment-measures-for-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cross-cultural-validity-of-functional-status-assessment-measures-for-rheumatoid-arthritis/