Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Antiphospholipid syndrome (APS) is a cause of pregnancy morbidity. Late pregnancy morbidity occurs in up to 25% of pregnant women with APS and may be the presenting manifestation of the autoimmune disease. We recently showed that aPL testing was performed in only 20% patients who displayed late pregnancy morbidity consistent with obstetrical APS. We aimed to determine the frequency of criteria and non-criteria anti-phospholipid (aPL) autoantibodies in patients admitted for unexplained fetal death (UFD), pre-eclampsia (PE) and/or fetal growth restriction (FGR).
Methods: All consecutive patients with UFD, PE and/or FGR followed in the department of Obstetrics, Bichat Hospital, University of Paris, Paris, between January 2019 and December 2021 were screened. Only patients with available serum stored from the index pregnancy were included. Patients with previously known APS or twin pregnancy were excluded. Testing for aPL autoantibodies included anti-cardiolipin (aCL), anti-β2GPI (aβ2GPI), anti-phosphatidylethanolamine (aPE), anti-phosphatidylserine/prothrombin (aPS/PT) IgG/IgM and anti-annexin V IgG. When available, placenta specimens were analysed by a pathologist blinded to the aPL status. All clinical characteristics, pregnancy features, and comorbidities were extracted from electronic medical records.
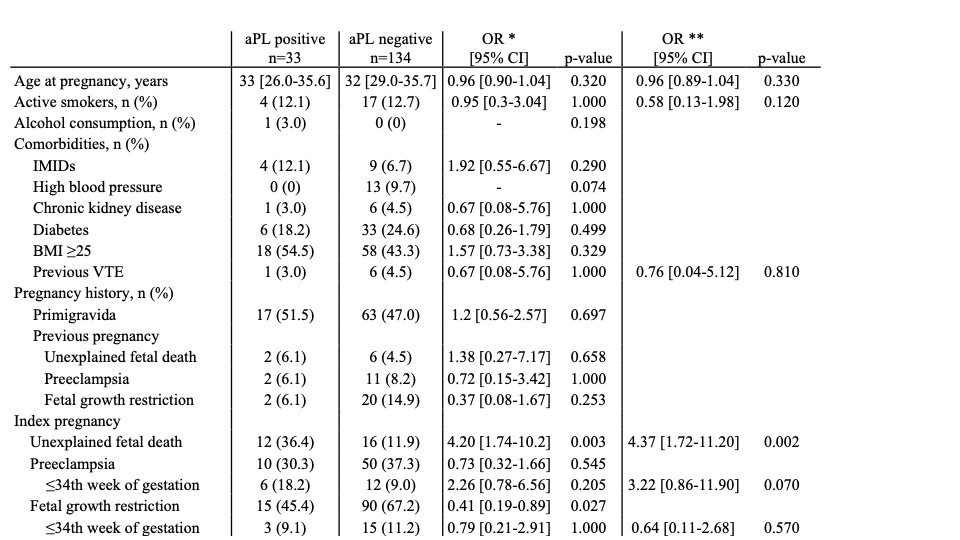
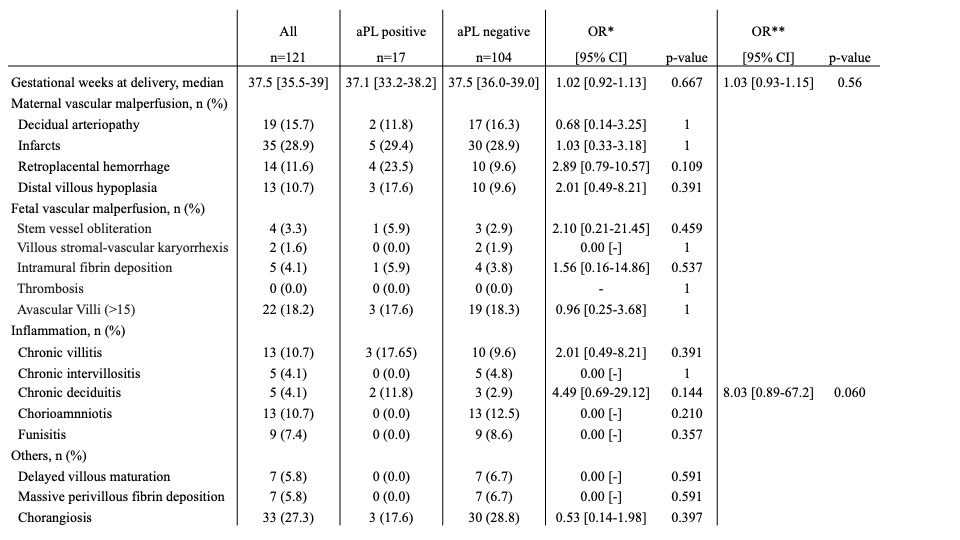
Results: Overall 167 (32 (28.8-35.7) years) patients with UFD (n=28; 16.8%), PE (n=60; 35.9%) and/or FGR (n=105; 62.9%) were screened for aPL autoantibodies. Moderate titers of aPL autoantibodies were detected in 33 (n=33/167, 19.8%) patients. aPL autoantibodies were non-criteria aPE IgG/IgM in most cases (n=28/33, 84.8%). aPS/PT IgG/IgM were found in 11 (n=11/33, 33.3%) cases and aCL or aβ2GP1 IgG/IgM in 4 (n=4/33, 12.1%). Multivariable logistic regression model showed that aPL autoantibodies were mostly associated with UFD (OR 4.37 [1.72-11.20], p=0.002), PE ≤34th week of gestation (3.22 [0.86-11.90], p=0.070) and chronic deciduitis (8.03 [0.89-67.2], p= 0.060) (Tables 1 and 2).
Conclusion: The frequency of aPL autoantibodies, mostly aPE, is high in patients with late pregnancy morbidity and may qualify obstetrical APS
Analysis was performed on variables collected in the 167 patients screened for aPL autoantibodies.
aPL, antiphospholipid autoantibodies; IMIDs, immune-mediated inflammatory diseases; VTE, Venous Thromboembolic Event; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Age is expressed as median [1st quartile- 3rd quartile].
*univariable and **multivariable logistic regression analysis.
Analysis was performed on variables collected in the 121 patients with available placenta specimens.
Small placenta weight for age defined as <25e percentile; retroplacental hemorrhage included retroplacental hemorrhage or basal decidual hematoma; distal villous hypoplasia defined by hypoxic ischemic villi > 50 %; chronic villitis included low grade, high grade or para basal villitis; massive perivillous fibrin deposition defined by perivillous fibrinoids deposits > 25 %
OR, odds ratio; confidence interval; aPL, antiphospholipid autoantibodies.
Age is expressed as median [1st quartile- 3rd quartile].
*univariable and **multivariable logistic regression analysis.
To cite this abstract in AMA style:
Goulenok T, Gros C, Mageau A, Barral T, Roland Nicaise P, Saint Frison M, Bucau M, Vivier V, Ferre V, Bourgeois Moine A, Papo T, Sacre K. Criteria and Non-criteria Antiphospholipid Autoantibodies Screening in Women with Unexplained Fetal Death, Pre-eclampsia And/or Fetal Growth Restriction: A Cross-sectional Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/criteria-and-non-criteria-antiphospholipid-autoantibodies-screening-in-women-with-unexplained-fetal-death-pre-eclampsia-and-or-fetal-growth-restriction-a-cross-sectional-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/criteria-and-non-criteria-antiphospholipid-autoantibodies-screening-in-women-with-unexplained-fetal-death-pre-eclampsia-and-or-fetal-growth-restriction-a-cross-sectional-study/