Session Information
Date: Tuesday, November 9, 2021
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II (1565–1583)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Ultrasound (US) has proven to be an excellent technique for detecting Calcium Pyrophosphate (CPP) deposits, however there are no grading systems that allow for a quantification and extent of deposition in patients. The only attempt was made on 2013 by Filippou et al. (Filippou G et al. Ann Rheum Dis 2013) but that score was never validated. The aim of this study is to create a scoring system for the quantification of CPP deposition at patient level according to the OMERACT framework.
Methods: According to the OMERACT methodology we performed a systematic literature review (SLR) aimed to estimate the prevalence of CPP deposition in peripheral joints by imaging in order to establish relevant joints for Calcium Pyrophosphate Deposition Disease (CPPD) monitoring [abstract submitted separately]. At the same time, a preliminary survey was circulated among the members of the OMERACT US – CPPD subgroup to collect their suggestions on the items to be included in the scoring system according to their personal experience. Subsequently, a Delphi survey was prepared and circulated between members of the OMERACT US – CPPD subgroup, including statements that reflected both the results of the SLR and of the preliminary survey. In total, 32 statements were created regarding the kind of scoring for single structures, the sites to be included, the final scoring at patient level, and the scanning technique. Participants were asked to reply on a Likert Scale and agreement was achieved when 4 and 5 grades reached 75% or more of concordance. In case of disagreement, new statements were proposed according to the members suggestions and proposed for voting in a consequent round.
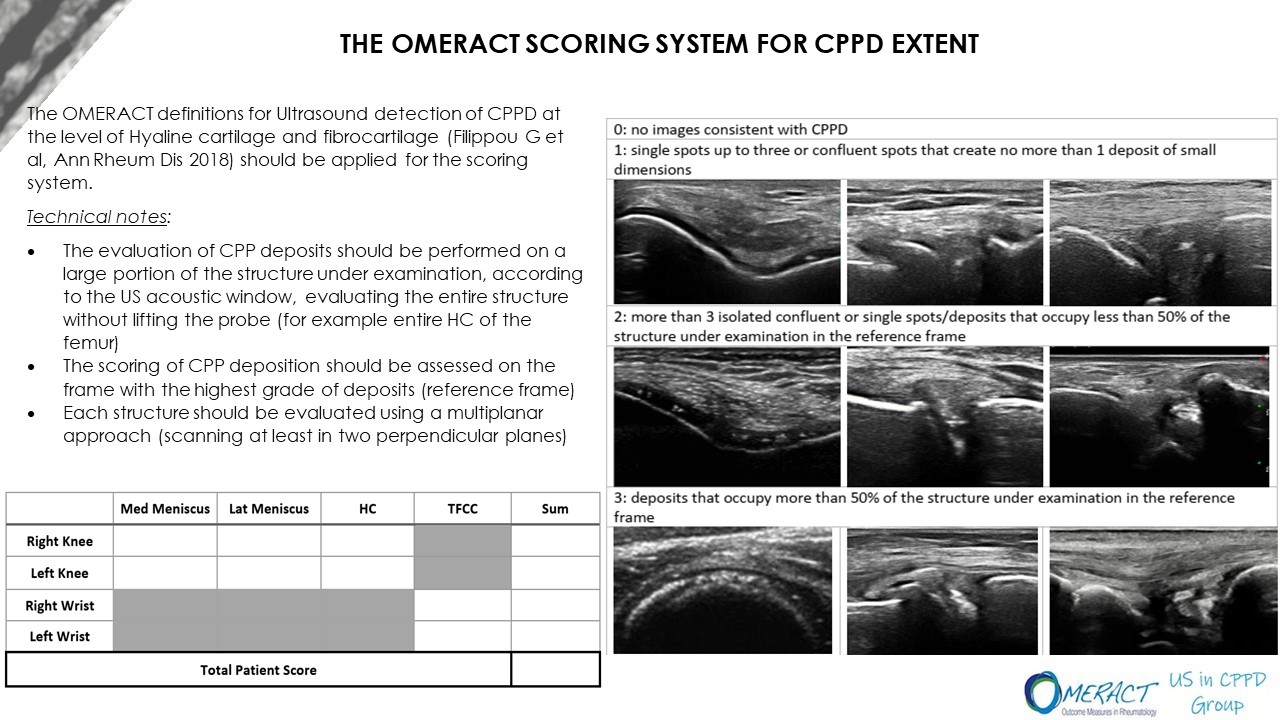
Results: 3 Delphi rounds were needed in order to reach agreement on all items. 32 participants out of 41 members replied at the first round, 26/32 at the second and 25/26 at the third round. 20 statements were approved in the first round, 3 at the second and 3 at the third. Statements in some cases were grouped together (for example the scale to use for the scoring) so once agreement was reached for one scoring the others were not proposed anymore. The experts decided to include only the knees (menisci and hyaline cartilage) and the triangular fibrocartilage of the wrists in the final score, using a four-grade scoring system (0-3). The final scoring with the definitions and the relative technical notes is represented in Figure 1.
Conclusion: This is the first attempt to create a scoring system for CPP crystals extent in patients affected by CPPD. In the next future the scoring will be assessed for reliability and hopefully released for use in clinical practice and research.
To cite this abstract in AMA style:
Sirotti S, Adinolfi A, Becce F, Cazenave T, Christiansen S, Cipolletta E, Delle Sedie A, Diaz Cortes M, Figus F, Filippucci E, Mandl P, MacCarter D, Moller I, Mortada M, Mouterde G, Naredo Sanchez M, Pineda C, Porta F, Schmidt W, Serban T, Terslev L, Vreju F, Wakefield R, Zufferey P, Sarzi-Puttini P, D'Agostino M, Damjanov N, Iagnocco A, Keen H, Filippou G. Creation of an Ultrasonographic Scoring System for CPPD Extent: Results from a Delphi Process by the OMERACT US Working Group – CPPD Subgroup [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/creation-of-an-ultrasonographic-scoring-system-for-cppd-extent-results-from-a-delphi-process-by-the-omeract-us-working-group-cppd-subgroup/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/creation-of-an-ultrasonographic-scoring-system-for-cppd-extent-results-from-a-delphi-process-by-the-omeract-us-working-group-cppd-subgroup/