Background/Purpose: Optimal COVID-19 vaccine responses are necessary to protect against severe infection. Patients with systemic rheumatic diseases (SRD) are at risk for not mounting adequate immune responses, at least partly because of treatment with immunosuppressive agents (ISA). Here we assess the effects of ISA on patients’ antibody (Ab) responses to COVID-19 vaccines.
Methods: This is a retrospective study based on chart data from patients with SRD in one of the authors’ (KK) practice, treated between 4/13/2021 and 8/6/2021. Patients who received full vaccination with either the Pfizer, Moderna, or Janssen vaccines and had received a SARS-CoV-2 Spike IgG Ab test after their final, non-booster, vaccine dose were considered (N=88). To allow for comparisons of Spike Ab titers between patients, only those who had the Siemens SARS-CoV-2 IgG (COV2G) assay were included (reference range < 1.0 (negative) and >20.0 IU) (N=60). All ISA received at the time of vaccinations were recorded. Treatment with rituximab (RTX) was considered even if given more than 6 months prior to vaccines. Absolute counts of peripheral B cells for patients on B cell agents [RTX and/or belimumab (BEL)] were available for most patients within 3 months from the time of vaccination, except for 6 patients on BEL. Chi-square, Fisher’s exact test, T-test, Mann-Whitney and linear regression were used. Statistical tests and graphs were performed with GraphPad Prism v9.1.2. An alpha of 0.05 was considered for statistical significance.
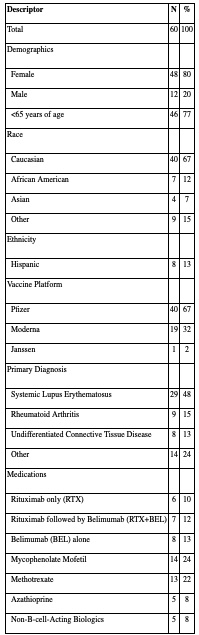
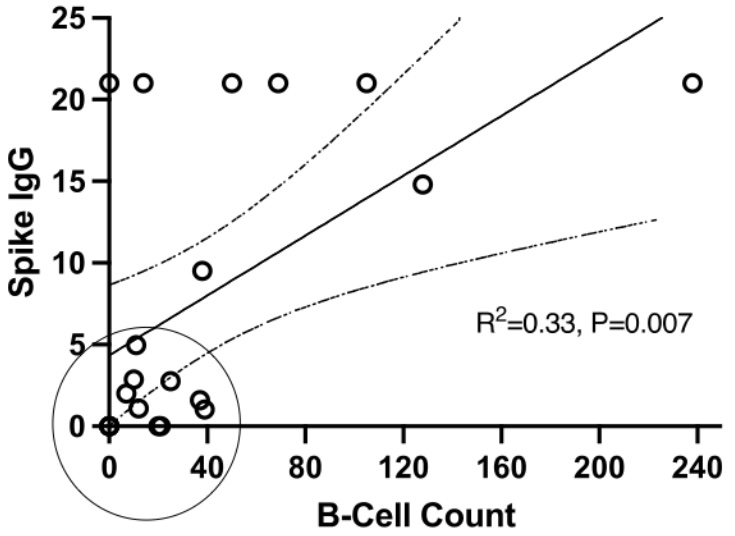
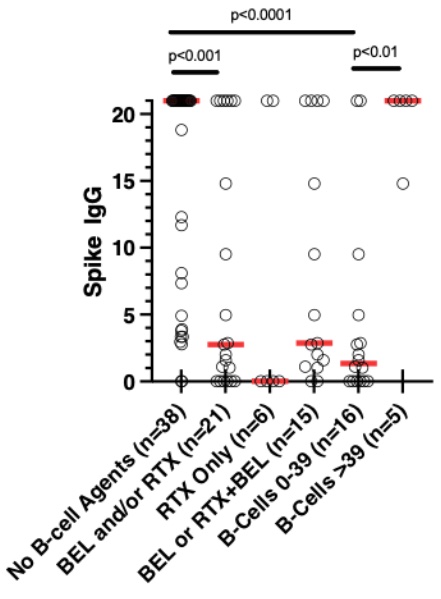
Results: The most common diagnosis in our study population of 60 was SLE (n=29) (Table 1). Most received the Pfizer vaccine (n=40). Median time between final vaccine and COV2G assay was 52.5 days (IQR 30-84.5). Nine patients (15%) had negative Spike Ab responses to the vaccine (recorded as 0), 30 (50%) had Ab titers of >20.0 (recorded as 21), and 21 (35%) had titers between 1.0 and 20.0 IU. The scatterplot of B cell counts in patients who received any B cell agent vs. their corresponding Spike IgG titers revealed a cluster of patients with very low Ab titers, all with absolute B cell counts below 40/μl (Figure 1; marked with a circle). Among 5 patients with undetectable B cell counts, 4 of them had negative Ab responses. B cell treatment groups included RTX only (n=6; of whom 2 within 6 months prior to vaccine), RTX at least 6 months prior to vaccine followed by BEL (n=7), and BEL only (n=8). Patients who received any B cell treatment had much lower Ab responses compared to those who did not (Figure 2). Interestingly, patients who achieved B-cell counts < 40 around the time of vaccination (n=16) had much lower spike IgG titers than those patients with B-cell counts of ≥40 (n=5), independent of the B cell agent used. We detected no significant effect in Ab responses attributable to other ISA or biologic agents, but we found that vaccination with Moderna was associated with higher Spike IgG titers than with Pfizer (p=0.0386).
Conclusion: Both RTX and BEL, significantly inhibited Ab responses to COVID-19 vaccines compared to other ISA. For the most part, the lower the B cell count, the greater the association. Considering B cell counts may better inform decisions regarding timing of vaccination in SRD patients on RTX/BEL, as well as ISA dose modifications around vaccination dates.
To cite this abstract in AMA style:
Kirou K, Zhang-Sun J. COVID-19 Vaccine Antibody Responses in Patients Treated with B-Cell Agents Depend on B-Cell Counts at Time of Vaccine [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/covid-19-vaccine-antibody-responses-in-patients-treated-with-b-cell-agents-depend-on-b-cell-counts-at-time-of-vaccine/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-vaccine-antibody-responses-in-patients-treated-with-b-cell-agents-depend-on-b-cell-counts-at-time-of-vaccine/