Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapies have attenuated vaccine responses and are prone to severe infections. Knowledge of COVID-19 outcome following vaccine and hybrid immunity, and identification of protective anti-Spike antibody concentrations are important to further develop vaccine strategies in this vulnerable patient group. The objectives of this study were to investigate the outcomes of COVID-19 in a large cohort of IMID patients compared to healthy controls, and in association to anti-Spike antibody concentrations and vaccine status.
Methods: In this prospective observational study, adult patients with IMID on immunosuppressive therapies were followed from February 15., 2021, to February 15., 2023. Throughout the study, patients and controls reported data regarding COVID-19 through questionnairs. COVID-19 related hospital admissions were recorded from The Norwegian Patient Registry. Anti-Spike antibodies were assessed 2-4 weeks following vaccination and COVID-19.
Results: A total of 1729 IMID patients with a documented COVID-19 history were included in the present analyses. 1140 (66%) of patients and 236 of 350 (67%) healthy controls (HC) reported COVID-19,the majority (85%) within the Omicron era. COVID-19 reinfection was reported in in 141 (12%) patients and 32 (14%) HC (p=0.66).
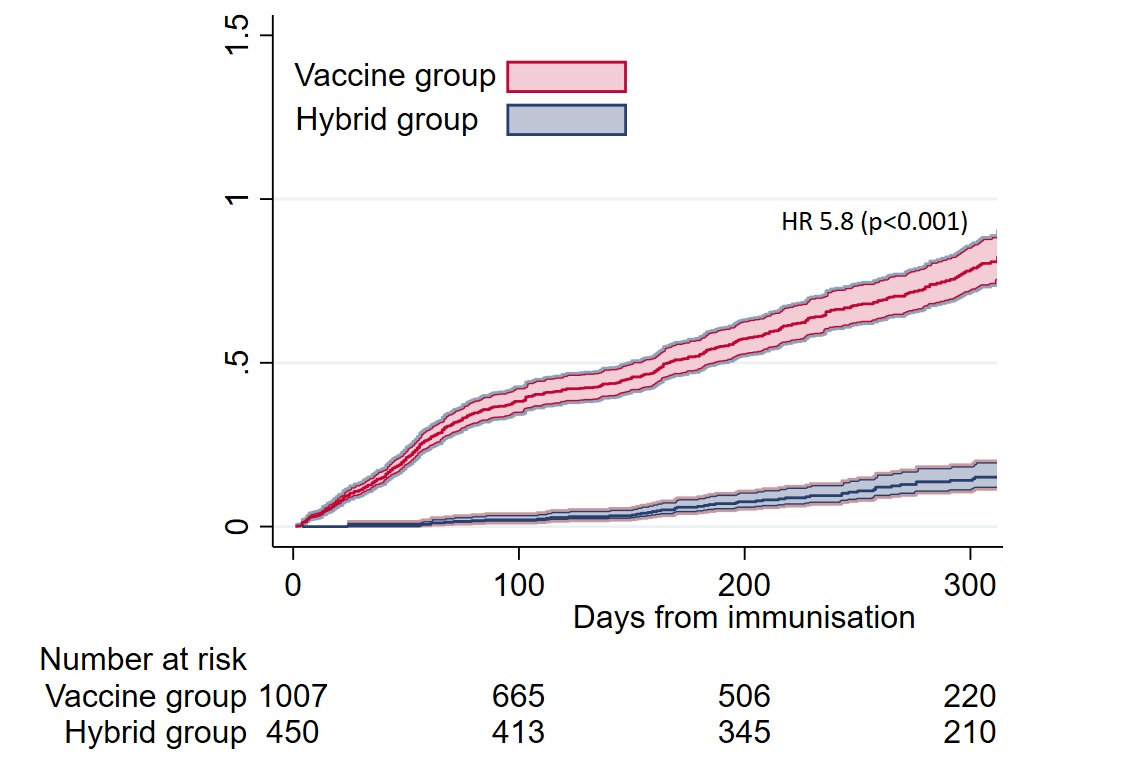
Compared to hybrid immunity (COVID-19 after a 3rd dose), the risk of COVID-19 was six times higher following vaccine series only (HR 5.8 (95% CI [4.4, 7.7]), p< 0.001) (Figure). Anti-Spike antibody concentrations < 6000 BAU/ml were predictive of COVID-19 after three (HR 1.4 (95% CI [1.1, 1.74]), p=0.01) and four vaccine doses (HR 1.3 (95% CI [1.02, 1.6]), p=0.04) and in hybrid immunity (HR 4.5 (95% CI [1·9, 10·7]), p=0.001).
In the entire cohort, the median anti-Spike antibody concentration after the 2nd vaccine dose was 2114 BAU/ml (732, 5749), after the 3rd dose 5897 BAU/ml (1939, 9761), after the 4th dose 7924 BAU/ml (3785, 15049) and following hybrid immunity 23505 BAU/ml (11423, 37007).
Hospitalisation due to COVID-19 (severe disease) occurred in 22 (2%) patients, (9 (41%) before any vaccination) and no HC. Four patients were admitted to intensive care. Prior to hospitalisation, the median anti-Spike antibody concentration was 444 BAU/ml (IQR 31-1634). Hospitalised patients with severe disease were older than non-hospitalised (median age 61 years (IQR 48-74) vs. 54 (42-64), p=0.04) and had a higher frequency of comorbidities (19/22 (86%) vs. 474/1118 (42%), p=0.02). No COVID-19 related deaths occurred.
Prolonged COVID-19 (symptoms >14 days) were reported by201 (18%) patients compared to 29 (12%) HC (p=0.05). The risk of prolonged disease was higher in the vaccine group compared to the hybrid group (HR 10.7 (95 % CI [4.8, 23.8]) p< 0.001)
Conclusion: IMID patients and healthy controls had a comparable occurrence of COVID-19, prolonged disease and reinfection. However, severe disease developed only in the patient group. Hybrid immunity protects against COVID-19 and prolonged disease. A high anti-Spike antibody concentration protects against COVID-19, supporting the role of repeated vaccination in IMID patients on immunosuppressive therapy.
To cite this abstract in AMA style:
Ørbo H, Jyssum I, Sexton J, Tveter A, Christensen I, Bjørlykke K, Kro G, Kvien T, Munthe L, Grødeland G, Mjaaland S, Haavardsholm E, Vaage J, Jørgensen K, Provan S, Syversen S, Goll G. COVID-19 Outcome and Association to Anti-Spike Antibody Levels in Patients on Immunosuppressive Therapy; A Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/covid-19-outcome-and-association-to-anti-spike-antibody-levels-in-patients-on-immunosuppressive-therapy-a-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-outcome-and-association-to-anti-spike-antibody-levels-in-patients-on-immunosuppressive-therapy-a-prospective-cohort-study/