Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Vaccines targeting the SARSCo-V2 virus protect against severe COVID-19 infection. Some immunosuppressive therapies impair SARSCo-V2 vaccine mediated immunogenicity and may increase the risk of severe COVID-19 infection even after vaccination. In people with Immune Mediated Inflammatory Diseases (IMIDs) who received at least one SARSCo-V2 vaccine, we aimed to describe the symptom profile and severity of COVID-19 infections.
Methods: As part of a single-center prospective observational cohort study of patients with diagnosed IMIDs who received SARSCo-V2 vaccines, we collected self-reported data regarding COVID-19 infection (test-confirmed vs suspected vs none), COVID-19 infection severity (ambulatory management, hospitalization, death), infection symptom profile, and risks for SARSCo-V2 virus exposure. Infection symptoms were categorized as constitutional (fever, fatigue), respiratory (rhinorrhea, sore throat, dyspnea, cough, chest pain), gastrointestinal (abdominal pain, nausea, emesis, diarrhea, loss of appetite), neurologic (headache, loss of taste/smell), and dermatologic (rash). IMID treatment was categorized as none vs immunomodulators, vs immunosuppressants, vs biologics/small molecules alone or in combinations. Participants provided blood samples 1, 3, and/or 6 months post each vaccination which were tested for anti-nucleocapsid (NC) IgG reflecting infection mediated immunogenicity and anti -Spike (S), and -receptor binding domain (RBD) IgG reflecting vaccine mediated immunogenicity. We describe the profile of COVID-19 infections across IMIDs.
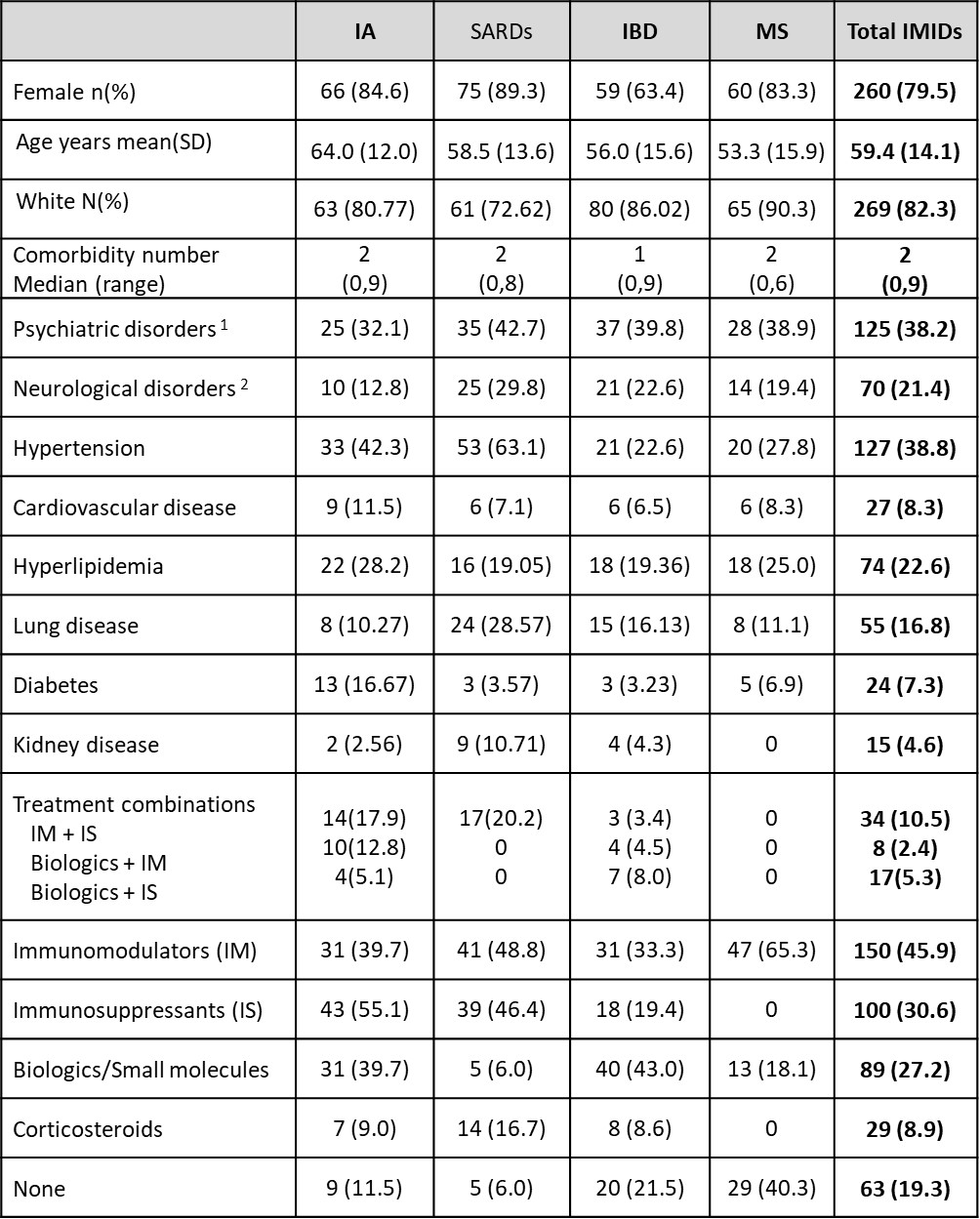
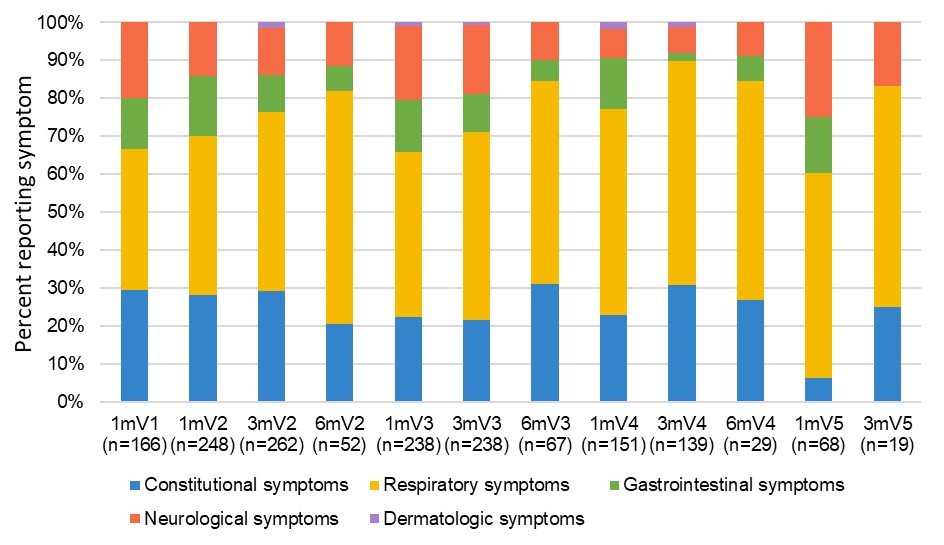
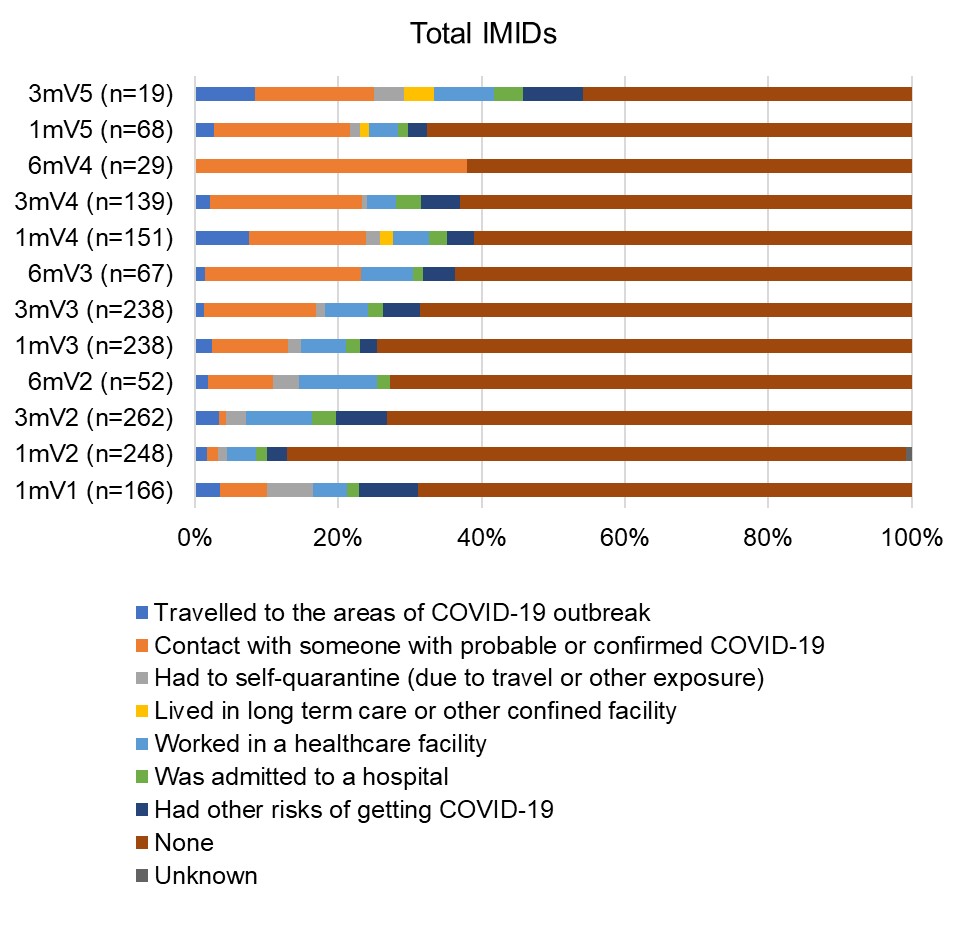
Results: COVID-19 self-reported infection data was available for 322 participants [Inflammatory Arthritis (IA) N=78; Systemic Autoimmune Rheumatic Diseases (SARDs) N=84; Inflammatory Bowel Disease N=88; Multiple Sclerosis N=72] who were predominantly female (79.8%) white (82.0%) with mean (standard deviation-SD) age 58.3(14.2) years and received a median (range) of 4 (1,5) vaccines (Table). Test-confirmed infections were reported by 64 (20%) participants (p=NS across IMIDs and sexes), viral symptoms by 109 (33.9%), no symptoms by 148 ( 46%). Those reporting infection were younger than those without infection [(median (interquartile range) years 59.0 (24.9) vs 62.2 (18.9) p=0.03]. Eighteen infections were seronegative for anti-NC a median of 33 days (range 3-178) post diagnosis. Most infections were mild, but 5 people were hospitalized and 3 died from COVID-19 (2 IA, 1 SARDs, age >75 years, with comorbidities and on biologics or IS; 2 were seronegative for anti-S and anti-RBD after 1 or 2 vaccines; 1 had low titers after 3 vaccines). COVID-19 symptoms were similar across IMIDs with constitutional and respiratory symptoms being most common (Figure 1). Of 85 new anti-NC positive infections, 26 were asymptomatic (p=NS across IMIDs). Most participants reported having no risk exposures to SARSCoV2 over the course of the study (Figure 2).
Conclusion: The symptom profile of COVID-19 is similar across IMIDs and to the general population. A subset of people with IMIDs may not generate robust anti-NC responses to documented COVID-19 infection. This may impact the reliability of surveillance studies relying on these assays.
IA= Inflammatory arthritis; CTD=Connective tissue disease; IBD=Inflammatory bowel disease; IMIDs=Immune Mediated Inflammatory Diseases
MSK=musculoskeletal. 1. depression, anxiety, bipolar disorder, schizophrenia; 2. migraine, epilepsy, transient ischemic attack/stroke)
V1=vaccine 1; V2=vaccine 2; V3=vaccine3; V4=vaccine 4; V5=vaccine 5
Symptom categories adapted from: Reaney, M., et al. Development of an Item Bank to Assess Patient-Reported Outcomes: Signs, Symptoms, and Impacts of COVID_19. Patient 15, 703–713 (2022).
To cite this abstract in AMA style:
Shcholok T, Bernstein C, Card C, Marrie R, Mesa C, Kim J, Hitchon C. COVID-19 Infection in People with Immune Mediated Inflammatory Diseases Who Received SARSCo-V2 Vaccines [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/covid-19-infection-in-people-with-immune-mediated-inflammatory-diseases-who-received-sarsco-v2-vaccines/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-infection-in-people-with-immune-mediated-inflammatory-diseases-who-received-sarsco-v2-vaccines/