Session Information
Date: Sunday, November 8, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Comorbidities
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with systemic lupus erythematosus (SLE) represent a unique population in considering risk for coronavirus disease 2019 (COVID-19) with biologic, genetic, demographic, clinical and treatment issues all at play. By the nature of their chronic inflammatory autoimmune condition and regular use of immunosuppressive medications, these individuals would traditionally be considered at high risk of contracting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and having a worse prognosis. Accordingly, we aimed to characterize patients with SLE affected by COVID-19 in New York City (NYC) and analyze associations of comorbidities and medications on outcomes.
Methods: Patients with SLE and COVID-19 (confirmed by RT-PCR testing), were identified through a longitudinal survey of an established NYU lupus cohort, query of New York University Langone Health and Bellevue Hospitals systems and referrals from rheumatologists at those institutions. All patients were age 18 or older and met SLE classification criteria or carried a rheumatologist’s diagnosis of SLE. Only English-, Spanish- or Mandarin-speaking patients were included in the study. Data were prospectively collected via a web-based questionnaire and review of electronic medical records. Baseline characteristics and medications were compared between the hospitalized and ambulatory patients with COVID-19. A logistic regression analysis was performed to identify independent predictors of hospital admission.
Results: A total of 41 SLE patients were confirmed COVID-19 positive by RT-PCR. The patients were predominantly female and encompassed the major racial/ethnic demographics seen in NYC. The most common symptoms of COVID-19+ patients were cough (78.4%), fever (64.9%), and shortness of breath (64.9%). Of those SLE patients with COVID-19, 24 (59%) were hospitalized, 4 required ICU level of care, and 4 died, all of hypoxic respiratory failure, Table 1. Hospitalized patients tended to be older, non-white, Hispanic, and have higher BMI, antiphospholipid syndrome, a history of lupus nephritis and at least one medical comorbidity, Table 2. There was no difference between the groups in use of hydroxychloroquine, systemic steroids or immunosuppressants. Logistic regression analysis identified the following independent predictors of being hospitalized with COVID-19: race (OR = 7.78 for non-white vs. white; 95% CI: 1.13 to 53.58; p=0.037), the presence of at least one comorbidity (OR=4.66; 95% CI: 1.02 to 21.20; p=0.047), and BMI (OR = 1.08 per increase in kg/m2; 95% CI: 0.99 to 1.18; p=0.096).
Conclusion: Patients with SLE and COVID-19 have a high rate of hospitalization but similar mortality rate to the general population in NYC. Risk factors such as non-white race, higher BMI, and the presence of one or more comorbidities were identified as independent predictors of hospitalization in SLE patients who develop COVID-19. The use of hydroxychloroquine and immunosuppressants did not appear to influence the outcomes of patients with SLE in the setting of COVID-19. Further studies are needed to understand additional risk factors for poor COVID-19 outcomes in patients with SLE.
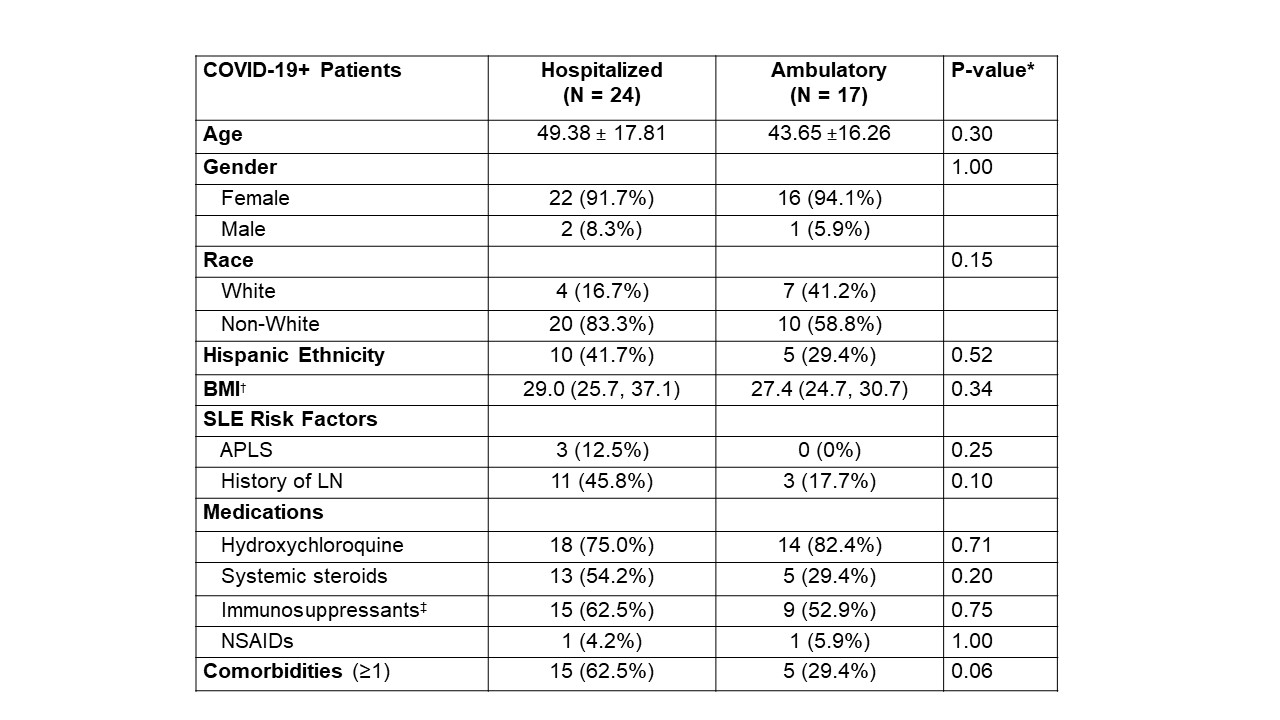 Table 2. Comparison of hospitalized and ambulatory SLE patients with confirmed COVID-19. Values are expressed as % (N) for categorical variables and mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables. * Categorical variables compared using Fisher’s exact test; continuous variables compared using the two-sample T-test or Mann Whitney U Test. Age: T-test; BMI: Mann Whitney U Test (chosen by whichever gave more conservative p-value). † Median (IQR); N=23 for Hospitalized group ‡ Immunosuppressants include non-biologic agents (azathioprine, cyclophosphamide, mycophenolate mofetil, mycophenolic acid, sirolimus, tacrolimus) and biologic agents (anakinra, abatacept, belimumab, rituximab, tocilizumab). § Comorbidities refers to at least one of the following: congestive heart failure, active malignancy, pregnancy, diabetes mellitus, asthma, chronic obstructive pulmonary disease. APLS, Antiphospholipid Syndrome; BMI, Body Mass Index; COVID-19, Coronavirus Disease 2019; COVID-19+, positive testing for SARS-CoV-2 by polymerase chain reaction; LN, Lupus Nephritis; SLE, Systemic Lupus Erythematosus.
Table 2. Comparison of hospitalized and ambulatory SLE patients with confirmed COVID-19. Values are expressed as % (N) for categorical variables and mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables. * Categorical variables compared using Fisher’s exact test; continuous variables compared using the two-sample T-test or Mann Whitney U Test. Age: T-test; BMI: Mann Whitney U Test (chosen by whichever gave more conservative p-value). † Median (IQR); N=23 for Hospitalized group ‡ Immunosuppressants include non-biologic agents (azathioprine, cyclophosphamide, mycophenolate mofetil, mycophenolic acid, sirolimus, tacrolimus) and biologic agents (anakinra, abatacept, belimumab, rituximab, tocilizumab). § Comorbidities refers to at least one of the following: congestive heart failure, active malignancy, pregnancy, diabetes mellitus, asthma, chronic obstructive pulmonary disease. APLS, Antiphospholipid Syndrome; BMI, Body Mass Index; COVID-19, Coronavirus Disease 2019; COVID-19+, positive testing for SARS-CoV-2 by polymerase chain reaction; LN, Lupus Nephritis; SLE, Systemic Lupus Erythematosus.
To cite this abstract in AMA style:
Fernandez-Ruiz R, Masson M, Kim M, Myers B, Haberman R, Scher J, Castillo R, Guttmann A, Carlucci P, Deonaraine K, Golpanian M, Robins K, Chang M, Belmont H, Buyon J, Blazer A, Saxena A, Izmirly P. COVID-19 in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/covid-19-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-in-patients-with-systemic-lupus-erythematosus/

