Session Information
Date: Tuesday, November 7, 2017
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud's – Clinical Aspects and Therapeutics II
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Little is known about systemic sclerosis (SSc)-associated myopathy (SScAM) treatment. Intravenous immunoglobulin (IVIg) are used off-label in SScAM. Herein we aimed to evaluate the use of IVIg in SScAM.
Methods : We conducted a retrospective study of patients with SScAM followed between 1993 and 2017 who received IVIg in the department of internal medicine of Cochin University Hospital. All patients fulfilled the ACR/EULAR criteria for SSc. SScAM was defined as a histologically proven myopathy or the association of myalgia and/or muscle impairment associated with elevation of muscle enzymes or myositic alteration at MRI.
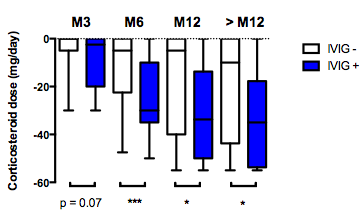
Results : Fifty-four patients were included in the study comprising 23 (42.6%) with limited cutaneous SSc and 27 (50%) with diffuse cutaneous SSc. SScAM occurred at a median [interquartile range (IQR)] time of 1 month [0 – 13] after the diagnosis of SSc. Thirty-six patients (66.7%) had muscle impairment, 29 (53.7%) had myalgia and 24 (44.4%) had dysphagia. Fifty-two patients (96.3%) had increased creatine kinase level. An electromyography was performed in 26 patients (48.1%) and was abnormal in 22 patients (84.6%). A MRI was performed in 13 patients (24.1%) and was pathological in 10 patients (76.9%). A muscle biopsy was performed in 52 patients (96.3%) and showed muscle involvement in 50 patients (92.6%), mainly inflammatory infiltrates (65.3% of the biopsies), and necrosis (57.4% of the biopsies). Twenty patients (37%) received IVIg. IVIg was initiated to treat SScAM because of worsening muscle involvement in 18 patients (90.0%) and was given in combination with corticosteroids in all patients. Adverse events (AEs) occurred in 7 patients (38.9%) including serious AEs in 2 patients (10%). After a median follow-up of 9 years [range 61-164], 18 patients (90%) had achieved clinical remission, every patient (100%) had achieved biological remission. When compared to patients who did not receive IVIG, there was no difference in remission rates, modified Rodnan skin score, lung function tests but patients who were treated with IVIg had a statistically significant higher decrease of corticosteroids at 6 and 12 months after treatment initiation (Figure 1).
Conclusion: This study shows the benefit of IVIg as adjunctive therapy, with an acceptable tolerance profile, and supports its use, especially as a corticosteroid-sparing agent, in SScAM.
Figure 1. Corticosteroid sparing after intravenous immunoglobulin infusion in patients with systemic sclerosis-associated myopathy
Box and whiskers show the minimum to the maximum corticosteroid decreased dose of 20 patients who received IVIG for systemic sclerosis-associated myopathy (SScAM) at 3, 6, 12 months and at the end of the follow-up period (>M12). Controls (n=34) were patients with SScAM who did not receive IVIG. Mann-Whitney test was used for comparisons. * p-value < 0.05, *** p-value < 0.001
To cite this abstract in AMA style:
Chaigne B, Rodeia S, Benmostefa N, Bérezné A, Cohen P, Regent A, Terrier B, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C, Mouthon L. Corticosteroid-Sparing Benefit of Intravenous Immunoglobulins in Systemic Sclerosis-Associated Inflammatory Myopathy: A Retrospective Study of 54 Patients [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/corticosteroid-sparing-benefit-of-intravenous-immunoglobulins-in-systemic-sclerosis-associated-inflammatory-myopathy-a-retrospective-study-of-54-patients/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/corticosteroid-sparing-benefit-of-intravenous-immunoglobulins-in-systemic-sclerosis-associated-inflammatory-myopathy-a-retrospective-study-of-54-patients/