Session Information
Date: Monday, November 11, 2019
Title: 4M096: Spondyloarthritis Including Psoriatic Arthritis – Clinical III: Miscellaneous (1818–1823)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic inflammatory disease that may lead to serious disability if not appropriately treated. Data on the effect of treatment discontinuation and retreatment in the case of flare are scant. Ixekizumab (IXE), a high-affinity IL-17A antagonist, has demonstrated consistent efficacy in PsA for up to 3 years.1 This study evaluated efficacy and safety of continuing vs. withdrawing IXE in PsA patients (pts) who achieved sustained minimal disease activity (MDA) on IXE and re-treating with IXE if required.
Methods: SPIRIT-P3 (NCT02584855) was a multicenter phase 3b study enrolling biologic-naïve pts with active PsA (diagnosis for ≥6 months, meeting Classification Criteria for Psoriatic Arthritis, ≥3/68 tender joints, ≥3/66 swollen joints) and previous inadequate response to conventional synthetic DMARDs (csDMARDs). Pts entered a 36-week, open-label (OL) treatment period with IXE every 2 weeks. Between Weeks 36–64, pts were randomized 1:1 to IXE or placebo (PBO) at the visit for which randomization criteria were met (sustained MDA for at least 4 visits over 3 consecutive months) and evaluated up to Week 104. Pts not meeting randomization criteria by Week 64 continued on IXE up to Week 104. Maintenance of treatment response was measured by the time to loss of sustained MDA (relapse) during the randomized withdrawal (RW) period. The proportion of pts who relapsed during the first 40 weeks of the RW period and time to regain MDA after re-treatment with IXE in relapsed pts were assessed. The Kaplan-Meier product limit method was used to estimate survival curves for time-to variables. Treatment comparisons were performed using a log-rank test adjusting for geographic region and csDMARD use as factors. Cumulative proportion of relapse was analyzed using a logistic regression model with treatment, geographic region, and csDMARD use as factors. Safety data were summarized for the entire study period for pts who received ≥1 dose of IXE.
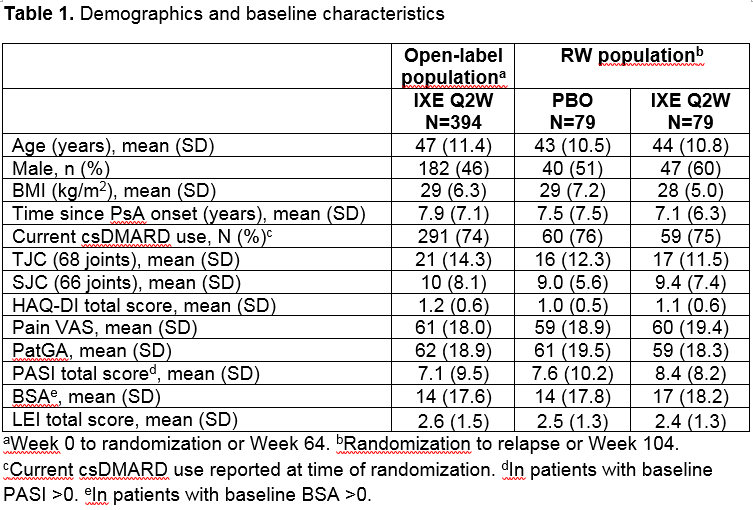
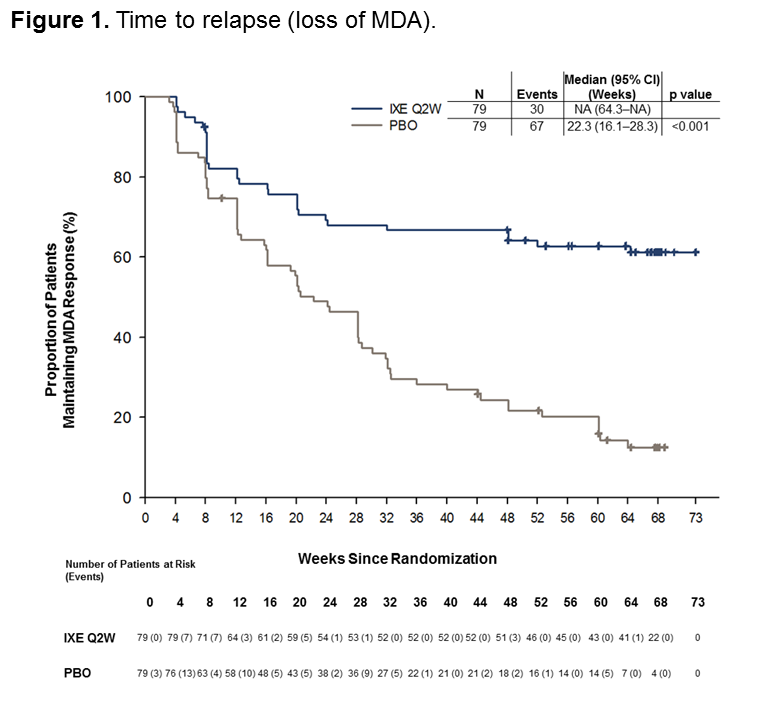
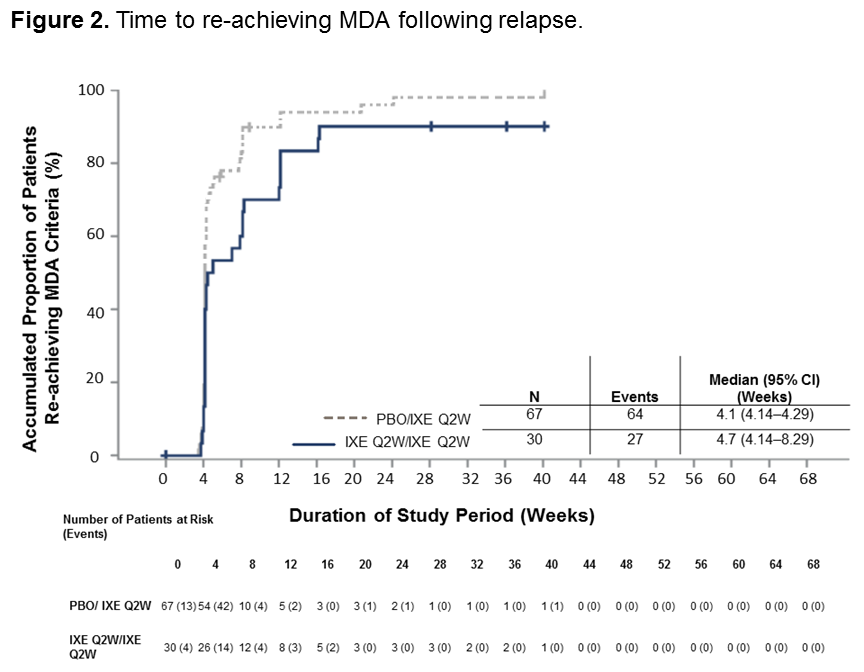
Results: A total of 394 pts entered into the OL treatment period; 158 (40%) achieved sustained MDA criteria and were randomized to IXE (N=79) or PBO (N=79). Baseline characteristics were similar between groups (Table 1). The time to relapse for pts on PBO was significantly shorter than for pts on IXE (p < 0.001) during the RW period, with a median time to relapse of 22.3 weeks in the PBO group (Figure 1). The cumulative relapse rate during the first 40 weeks of the RW period was 73% for PBO vs. 34% for IXE (p < 0.001). Of the patients who relapsed on PBO, 96% regained MDA following re-treatment with IXE. The median time to regain MDA was 4.1 weeks (95% CI 4.14–4.29) for PBO and 4.7 weeks (95% CI 4.14–8.29) for IXE (Figure 2). Safety data were consistent with previous IXE PsA studies with no unexpected safety signals.1
Conclusion: Continued IXE therapy was superior to PBO in maintaining MDA in biologic-naïve PsA pts who achieved sustained MDA on initial IXE treatment. A vast majority of pts who lost MDA after IXE withdrawal regained MDA with IXE re-treatment. Continuous IXE treatment is optimal for maintaining MDA; however, patients can regain MDA after re-treatment with IXE in case of treatment interruption.
1. Chandran V, et al. Ann Rheum Dis. 2018;77(Suppl 2): 385.
Time to relapse in weeks = -date of relapse – date of first injection of randomized dose of study treatment in the randomized double-blind withdrawal period +1-/7. Patients completing the withdrawal period without meeting relapse criteria were censored at the date of completion -the date of the last scheduled visit in the withdrawal period-. Patients without a date of completion or discontinuation were censored at the latest non-missing date from the following dates: date of last injection of study treatment in the withdrawal period and date of last attended visit in the withdrawal period.
The relapse population is defined as randomized patients who relapsed -no longer met criteria for MDA- after randomization and who received at least 1 dose of IXE after relapse.
To cite this abstract in AMA style:
Coates L, Pillai S, Zhang L, Adams D, Kerr L, Hojnik M, Gallo G, Valter I, Tahir H, Chandran V, Mease P, Kavanaugh A. Continuing versus Withdrawing Ixekizumab in Patients with Psoriatic Arthritis Who Achieved Sustained Minimal Disease Activity: Results from the SPIRIT-P3 Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/continuing-versus-withdrawing-ixekizumab-in-patients-with-psoriatic-arthritis-who-achieved-sustained-minimal-disease-activity-results-from-the-spirit-p3-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continuing-versus-withdrawing-ixekizumab-in-patients-with-psoriatic-arthritis-who-achieved-sustained-minimal-disease-activity-results-from-the-spirit-p3-study/