Session Information
Date: Monday, November 6, 2017
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Romosozumab (Romo) is a monoclonal antibody that binds sclerostin with a dual effect, increasing bone formation and decreasing bone resorption. In the primary analysis of the multicenter, double-blind, FRActure study in postmenopausal woMen with ostEoporosis (FRAME) (NCT01575834), women aged 55–90 (T-score ≤–2.5 at total hip [TH] or femoral neck), Romo significantly reduced vertebral and clinical fracture risk vs placebo (Pbo) at 12 months (M).1 A continued 75% new vertebral fracture and 33% clinical fracture relative risk reduction was observed at 24M in patients who initially received Romo, despite all patients receiving denosumab (DMAb) after 12M. BMD showed rapid and robust increases with Romo at the spine and hip, and these increases continued on transition to DMAb.1
Methods: In FRAME, patients received Pbo or Romo SC monthly for 12M, followed by open-label DMAb SC every 6M for 12M. Here, we report results through 36M after a further 12M of open-label DMAb. Key endpoints included patient incidence of new vertebral, clinical (nonvertebral plus symptomatic vertebral), and nonvertebral fracture, and BMD.
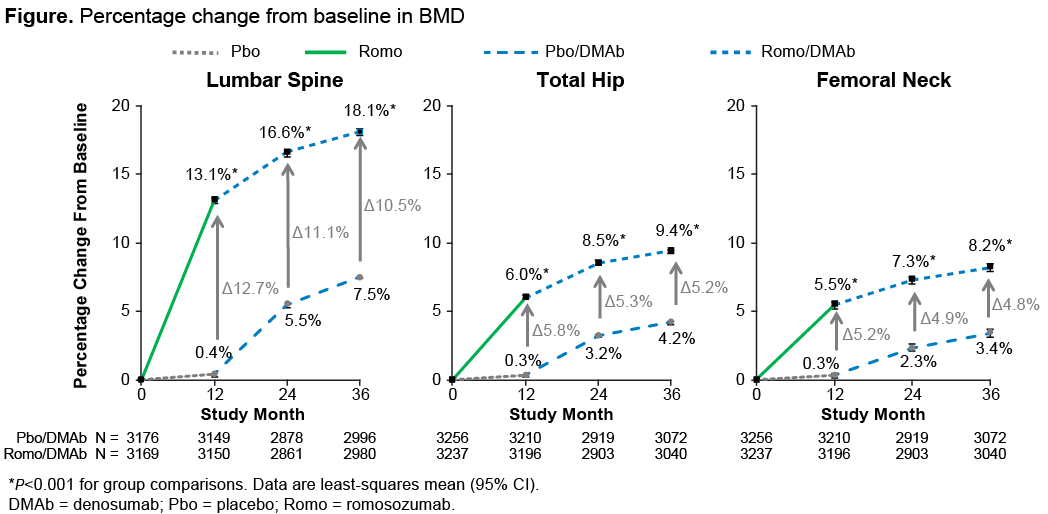
Results: Of the 7180 women enrolled, 5743 (80%) completed the 36M study (2892 Pbo followed by DMAb; 2851 Romo followed by DMAb). Through 36M, fracture risk reduction was observed for new vertebral, clinical, nonvertebral, and other predefined fracture endpoints in patients who received Romo followed by 24M of DMAb vs Pbo followed by 24M of DMAb (Table). At 36M, BMD continued to increase in the Romo followed by DMAb group to 18.1% (lumbar spine) and 9.4% (TH) (Figure). The mean differences in BMD in patients who received Romo in the first 12M compared with those initially on Pbo remained significant over the entire 36M (P<0.001). Adverse events were generally balanced between groups; no additional positively adjudicated cases of atypical femoral fracture or osteonecrosis of the jaw were reported since reporting the 24M data.1
Conclusion: In postmenopausal women with osteoporosis, Romo followed by DMAb was well tolerated and reduced new vertebral, clinical, and nonvertebral fracture risk vs Pbo followed by DMAb for 24M. BMD continued to increase in both groups. BMD in patients treated with Romo in the first 12M remained significantly higher than in those who initially received Pbo despite all patients receiving DMAb for 24M, underscoring the important foundational effect of Romo. The sequence of Romo followed by DMAb will be a promising treatment regimen for postmenopausal women with osteoporosis.
1Cosman NEJM 2016
To cite this abstract in AMA style:
Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi J, Miyauchi A, Gielen E, Milmont CE, Libanati C, Grauer A. Continued Fracture Risk Reduction after 12 Months of Romosozumab Followed By Denosumab through 36 Months in the Extension of the Phase 3 Fracture Study in Postmenopausal Women with Osteoporosis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/continued-fracture-risk-reduction-after-12-months-of-romosozumab-followed-by-denosumab-through-36-months-in-the-extension-of-the-phase-3-fracture-study-in-postmenopausal-women-with-osteoporosis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continued-fracture-risk-reduction-after-12-months-of-romosozumab-followed-by-denosumab-through-36-months-in-the-extension-of-the-phase-3-fracture-study-in-postmenopausal-women-with-osteoporosis/