Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Immunomodulatory agents (IA) are widely used for the treatment of inflammatory rheumatic diseases (IRDs). Although IA are safe and effective, management of infections and infection risk remains an issue. It is unclear how physicians should act in case of an intercurrent infection. Most guidelines and Summaries of Product Characteristics recommend temporary interruption, but based on indirect evidence, continuation of IA might be an equally safe and perhaps better treatment option regarding infection outcome. The aim of this study was to assess the effect of continuation of IA versus temporary interruption in case of an infection.
Methods: This study is a two arm, open-label, pragmatic, explorative randomized controlled strategy study among IRD patients using IA in The Netherlands. At baseline, participants were randomized 1:1 into continuation or temporary interruption of IA during their first clinically relevant infection, stratified for the use of TNF inhibitors, oral glucocorticoids and risk of severe COVID-19 (based on the categorization of the CDC). Primary outcome was the proportion of patients with a serious infection (requiring hospitalisation or intravenous treatment), analysed in the intention-to-treat (ITT) population using the Cochran-Mantel-Haenszel risk difference and confidence interval. The ITT population consisted of all patients who experienced at least one clinically relevant infection. Secondary analyses included Complier Average Causal Effect (CACE) analyses, taking into account non-compliance. This trial is registered in the Dutch Trial Register (NL8922).
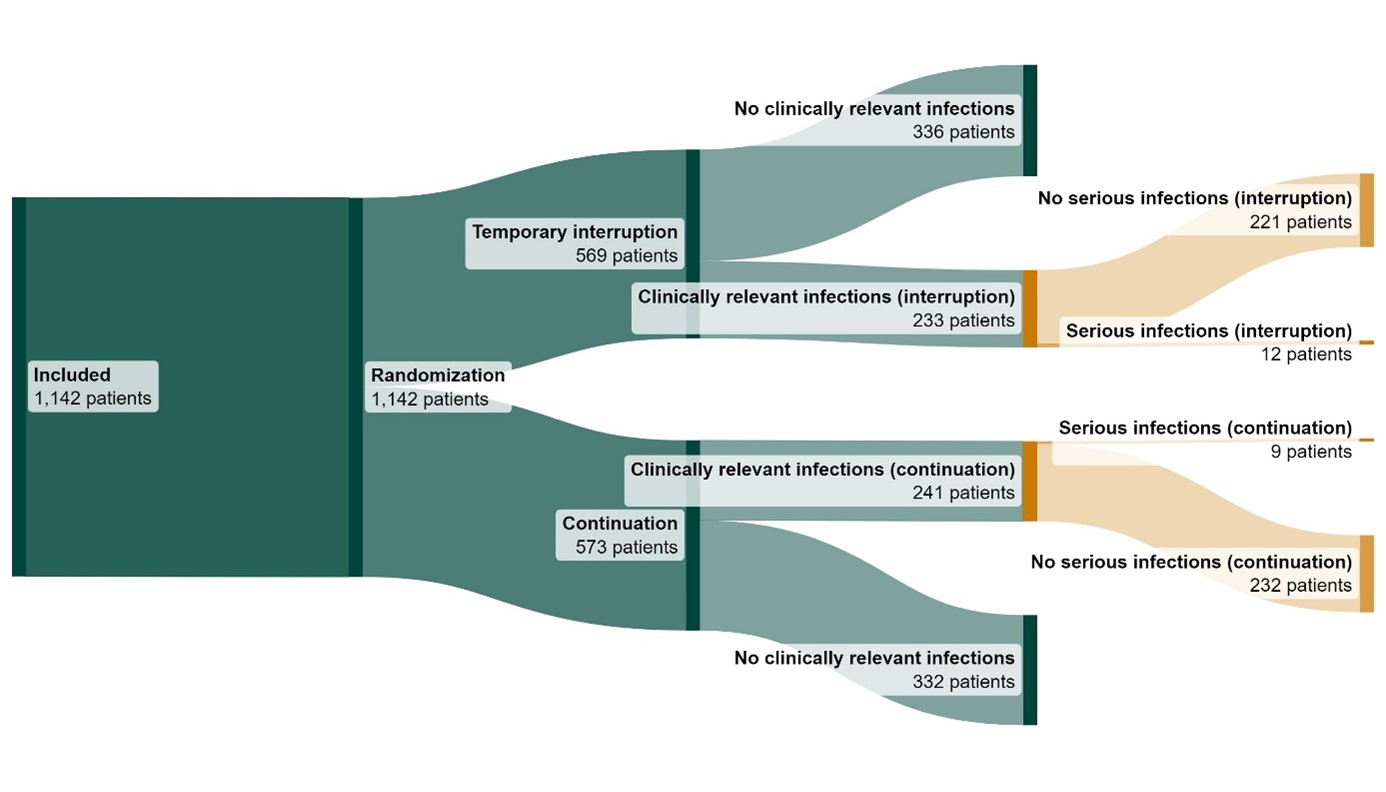
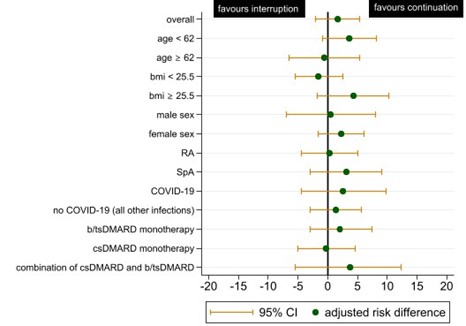
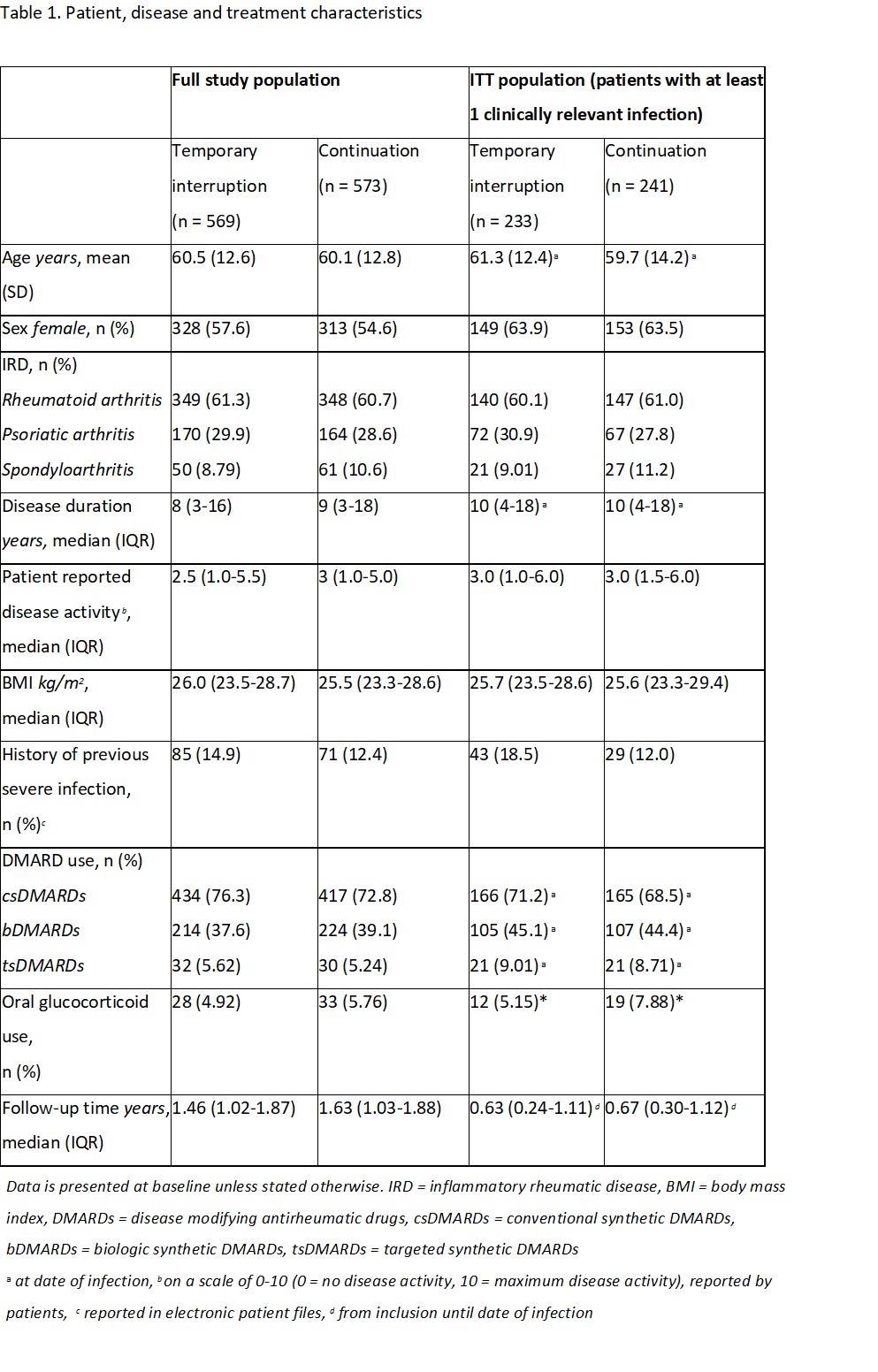
Results: In total, 1142 patients were included with a total follow-up time of 1667 patient years. The ITT population consisted of 474 patients. In table 1, patient, treatment and disease characteristics for the entire study population and the ITT population are shown. Serious infections occurred in 12/233 patients (5.15%) in the temporary interruption group and in 9/241 (3.73%) in the continuation group, resulting in an adjusted risk difference of 1.71% (95% CI -1.99 to 5.39) (figure 1). Subanalyses in different subsets of the study population showed similar adjusted risk differences (figure 2). The CACE method showed a risk difference of 4.51 (95% CI -7.32 to 16.34) favouring continuation.
Conclusion: Exploratively, we found similar infection outcomes between temporary interruption and continuation of IA during an infection. The findings of these study suggest that continuation of IA might be a safe option.
To cite this abstract in AMA style:
Opdam M, den Broeder N, van Crevel R, Schapink L, Raijmakers L, Broen J, Verhoef L, den Broeder A. Continuation versus Temporary Interruption of Immunomodulatory Agents in Case of an Infection in IRD Patients: Results of a Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/continuation-versus-temporary-interruption-of-immunomodulatory-agents-in-case-of-an-infection-in-ird-patients-results-of-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continuation-versus-temporary-interruption-of-immunomodulatory-agents-in-case-of-an-infection-in-ird-patients-results-of-a-randomized-controlled-trial/