Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The Routine Assessment of Patient Index Data (RAPID3) was developed and validated for rheumatoid arthritis (RA). Little data exist on the use of RAPID3 in psoriatic arthritis (PsA). The objective of this study was to assess the construct validity (the ability of an instrument to measure the concepts it attempts to measure) of RAPID3 through investigation of its correlation with other validated measures of disease activity, physical function, and life impact among patients with PsA.
Methods: Patients with PsA were enrolled in the Psoriatic Arthritis Research Consortium (PARC) between 2015-2016. PARC is a longitudinal observational cohort study conducted at four institutions: University of Pennsylvania, Cleveland Clinic, New York University, and University of Utah. Standardized data are collected and entered into a Research Electronic Data Capture database. All sites collected RAPID3 and provider-assessed outcomes, including tender and swollen joint counts, provider global assessment of overall health, and provider global assessment of arthritis. Three sites administered the Short Form 12 (SF12), and two sites collected the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale, Psoriatic Arthritis Quality of Life (PsAQoL) index, Dermatology Life Quality Index (DLQI), Work Limitations Questionnaire (WLQ), and Work Productivity and Activity Impairment (WPAI) questionnaire. Licenses have been obtained for the use of the SF-12 and the WLQ. Only baseline data were included in this cross-sectional analysis. Construct validity was assessed with SpearmanÕs correlation coefficients between RAPID3, other PROs, and provider-assessed disease activity measures.
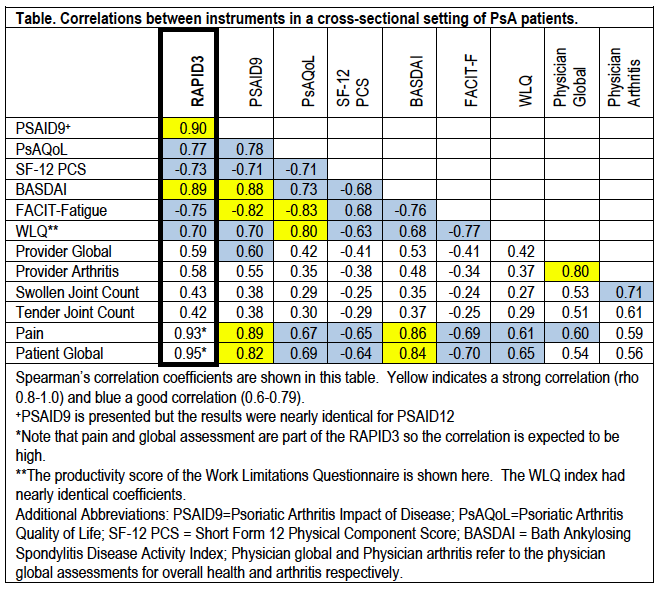
Results: Among 401 PARC participants with RAPID3 scores, 275 had SF12 scores, and 208 had full set of surveys. Of those with complete surveys, the mean age was 51.5 and 57% were female. The RAPID3 was highly correlated with the PSAID9 (r=0.90) and BASDAI (0.89) (Table). RAPID3 was also correlated with the SF12 physical component score (r=-0.73), the FACIT-F (r=0.75), the work limitations questionnaire (r=0.70), tender joint count (r=0.43), and swollen joint count (r=0.43).
Conclusion: RAPID3 has good construct validity in PsA and measures disease impact more broadly than physical function and pain. RAPID3 scores strongly correlated with the PSAID, a PRO with specific questions addressing many facets of life impact (e.g., pain, function, fatigue, sleep, coping, anxiety and depression). The moderate correlations between RAPID3 and provider-assessed outcomes demonstrated that patients interpreted their disease states differently than their providers.
To cite this abstract in AMA style:
Walsh JA, Willinger C, Husni ME, Reddy SM, Scher JU, Ogdie A. Construct Validity of RAPID3 for Measurement of Disease Activity in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/construct-validity-of-rapid3-for-measurement-of-disease-activity-in-psoriatic-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/construct-validity-of-rapid3-for-measurement-of-disease-activity-in-psoriatic-arthritis/