Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Apremilast (APR), an oral phosphodiesterase 4 inhibitor, regulates immune activity in psoriatic arthritis (PsA) patients. We evaluated the long-term safety of APR treatment for up to 4 years in subjects with active PsA despite prior conventional disease-modifying anti-rheumatic drugs (DMARDs) and/or biologics. Safety data were pooled from the phase 3 PALACE 1, 2, and 3 studies.
Methods: Subjects were randomized at baseline (1:1:1) to receive placebo (PBO), APR 30 mg BID (APR30), or APR 20 mg BID (APR20). PBO subjects were re-randomized to APR30 or APR20 at Week 16 (early escape) or Week 24. Double-blind APR treatment continued to Week 52; subjects could continue APR during an open-label, long-term treatment phase for up to 5 years treatment. Visits in years 2, 3, and 4 were scheduled at 13-week intervals. Safety was assessed at each visit throughout the study, and results are summarized here by exposure.
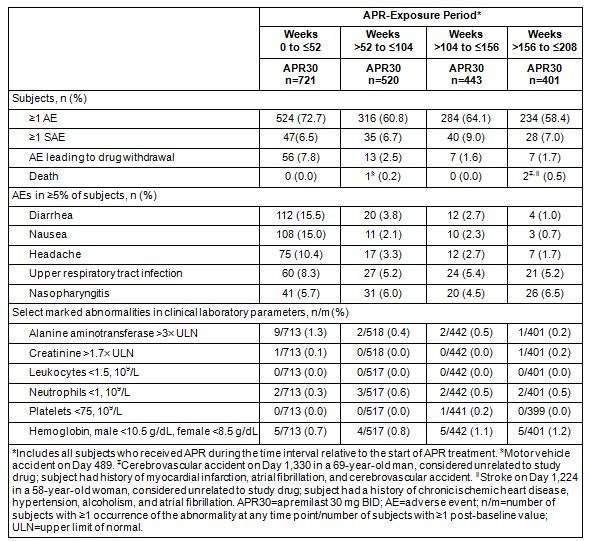
Results: A total of 1,493 subjects were randomized and received ≥1 dose of study medication (PBO: n=495; APR30: n=497; APR20: n=501). At the 4-year data cut, the numbers of subjects receiving APR30 and APR20 in each exposure period were 1,441 in Weeks 0 to ≤52, 1,028 in Weeks >52 to ≤104, 865 in Weeks >104 to ≤156, and 767 in Weeks >156 to ≤208. During the 0- to ≤52-week APR-exposure period, adverse events (AEs) occurring in ≥5% of APR30-exposed subjects were diarrhea, nausea, headache, upper respiratory tract infection, and nasopharyngitis (Table). Most diarrhea and nausea AEs were reported within the first 2 weeks of treatment and usually resolved within 4 weeks; the frequency of gastrointestinal AEs decreased with longer APR30 exposure, and the frequency of other common AEs either decreased or remained stable with prolonged exposure (Table). Most AEs were mild or moderate in severity. During Weeks >156 to ≤208 of APR exposure, the discontinuation rate due to AEs was 1.7% with APR30, and the rate of serious AEs (SAEs) was 7.0%, consistent with earlier periods; most SAEs occurred in 1 subject each. Rates were very low for major cardiac events, malignant neoplasms, and serious opportunistic infections, comparable to the first year of treatment. Rates of depression remained very low in Weeks >156 to ≤208. Marked laboratory abnormalities were infrequent, and most returned to baseline values with continued treatment.
Conclusion: APR30 demonstrated a favorable and consistent safety profile and was well tolerated for up to 208 weeks, marked by the lack of an increase in infection rates or a need for specific laboratory monitoring. The incidence of AEs remained stable or decreased with long-term exposure to APR30.
To cite this abstract in AMA style:
Mease PJ, Gladman DD, Gomez-Reino JJ, Hall S, Kavanaugh A, Lespessailles E, Schett G, Paris M, Teng L, Wollenhaupt J. Consistent Safety Profile with up to 4 Years of Apremilast Treatment: Analysis of Data from 1,493 Subjects with Psoriatic Arthritis in 3 Large, Phase III, Long-Term Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/consistent-safety-profile-with-up-to-4-years-of-apremilast-treatment-analysis-of-data-from-1493-subjects-with-psoriatic-arthritis-in-3-large-phase-iii-long-term-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistent-safety-profile-with-up-to-4-years-of-apremilast-treatment-analysis-of-data-from-1493-subjects-with-psoriatic-arthritis-in-3-large-phase-iii-long-term-studies/