Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pooled safety data from the psoriasis (PsO) and psoriatic arthritis (PsA) clinical trial programs of secukinumab (SEC), through approximately 1 year, have been reported previously.1,2 Specifically, the exposure-adjusted incidence rates (EAIRs) of adverse events (AEs) and serious adverse events (SAEs) for PsO and PsA were 252.9 and 7.8, and 210.3 and 9.0 per 100 patient (pt)-years, respectively. Here we present updated analyses following additional exposure (with a 25 Dec 2015 data cut-off), either from a larger study pool (PsO) and/or an extended follow-up period (PsO, PsA).
Methods: The updated PsO pool consisted of 4 Phase II and 9 Phase III studies in pts with moderate-to-severe plaque PsO, including 2 active comparator studies with etanercept (ETN) and ustekinumab (UST), respectively; the PsA pool consisted of 2 PBO-controlled Phase III studies in pts with active PsA. SEC doses differed in the studies and included intravenous (3–10 mg/kg) or subcutaneous (75–300 mg) loading, followed by subcutaneous maintenance dosing (300, 150, or 75 mg). PBO pts were re-randomized to SEC between 12–24 weeks in the various studies. EAIRs were calculated to minimize the impact of between-group differences in treatment exposure.
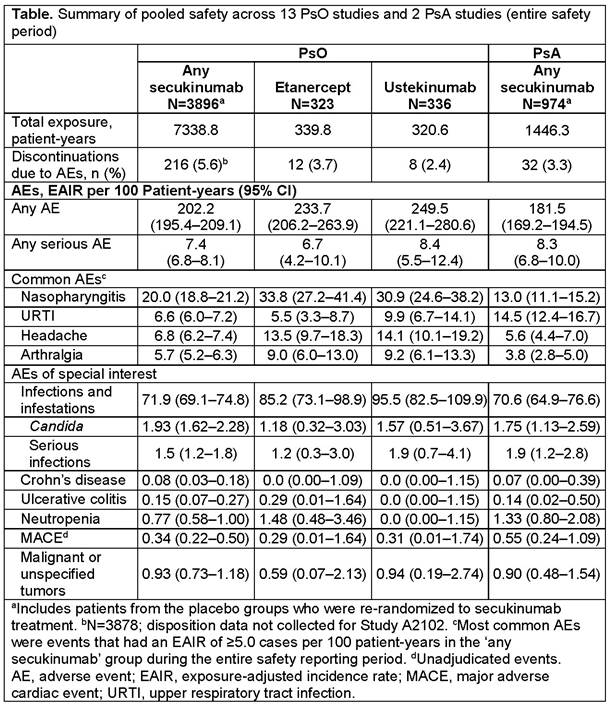
Results: In both the PsO and PsA pools, the most frequently reported AEs with SEC were non-serious infections, including nasopharyngitis and upper respiratory tract infection, headache, and arthralgia (Table). The EAIRs of AEs of special interest, including Crohn’s disease, Candida infections, serious infections, neutropenia, major adverse cardiac events, and malignancy, with SEC (reported in the Table) were similar across PsO and PsA studies, and comparable to those reported previously.1,2
Conclusion: Treatment with SEC was well tolerated in PsO and PsA patients. This longer-term safety assessment was consistent with previous reports,1,2 and did not identify any new safety signals. The long-term safety profile of SEC was comparable across PsO and PsA patients, allowing a broader understanding of the safety of SEC in these two closely related disease populations. References: 1. Van de Kerkhof PCM, et al. J Am Acad Dermatol 2016;75:83–98; 2. Mease PJ, et al. Arthritis Rheumatol 2015; 67 (suppl 10):A2886
To cite this abstract in AMA style:
Mease PJ, McInnes IB, Reich K, Andersson M, Tao A, Fox T, Karyekar C. Consistent Safety and Tolerability of Secukinumab over Long-Term Exposure in Patients with Active Psoriatic Arthritis and Moderate to Severe Plaque Psoriasis: Updated Pooled Safety Analyses [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/consistent-safety-and-tolerability-of-secukinumab-over-long-term-exposure-in-patients-with-active-psoriatic-arthritis-and-moderate-to-severe-plaque-psoriasis-updated-pooled-safety-analyses/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistent-safety-and-tolerability-of-secukinumab-over-long-term-exposure-in-patients-with-active-psoriatic-arthritis-and-moderate-to-severe-plaque-psoriasis-updated-pooled-safety-analyses/