Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Some patients with rheumatoid arthritis (RA) receiving tumor necrosis factor-inhibitors (TNF-Is) show inadequate response to TNF-Is. But it has not been clarified what is better as the second biological agents by clinical trials, so observational study is necessary. We conducted a retrospective cohort study, especially focused on the difference of the drug retention rates between bio-naïve patients and switching patients.
Methods: We retrospectively reviewed the medical records of patients with RA, who were administered biologics (tocilizumab (TCZ) or TNF-Is (infliximab, etanercept, adalimumab, and golimumab)) in our institute from September 1999 to April 2012, and analyzed the retention rates and causes of the discontinuation of the biologics. Kaplan-Meier estimate and log-rank test were used to analyze the differences of the drug continuation rates between bio-naïve patients and switching patients.
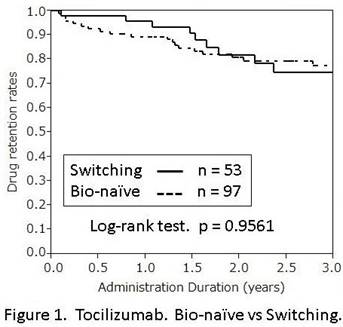
Results: TCZ was administered to 97 bio-naïve patients and 53 switching patients. TNF-Is were administered to 318 bio-naïve patients and 89 switching patients. Median (range) administration duration of TCZ was 2.54 years (0.08-12.6) for bio-naïve patients and 1.78 years (0.08-3.87) for switching patients, respectively. And that of TNF-Is was 1.87 years (0-11.3) for bio-naïve patients and 0.94 years (0-5.95) for switching patients, respectively. Kaplan-Meier Curves of the time to discontinuation due to unfavorable causes were shown (Figure 1, 2). Regarding TCZ, there was no significant difference of the drug retention rates between bio-naïve patients and switching patients (p = 0.9561), while regarding TNF-Is, there was a significant difference (p = 0.0037). To clarify the reason for this difference between TCZ and TNF-Is, we conducted Kapran-Meier estimate for cause-specific rates of discontinuation. About TCZ, the cumulative occurrence of discontinuation due to lack or loss of efficacy and adverse events were not statistically different between bio-naïve patients and switching patients (p = 0.1376, p = 0.3683, respectively). But about TNF-Is, discontinuation due to lack or loss of efficacy was significantly more in switching patients than in bio-naïve patients (p < 0.0001), while cumulative discontinuation due to adverse events was not statistically different (p=0.8334).
Conclusion: TCZ showed high tolerability in both bio-naïve patients and switching patients, while TNF-Is showed significantly lower tolerability in switching patients than in bio-naïve patients. This high retention rate of TCZ for switching patients was considered to be due to durability of its efficacy.
Disclosure:
Y. Hishitani,
None;
Y. Shima,
None;
T. Hirano,
None;
K. Hagihara,
None;
K. Ebina,
None;
Y. Kunugiza,
None;
K. Shi,
None;
M. Narazaki,
None;
A. Ogata,
Chugai,
5;
T. Tomita,
None;
T. Tanaka,
None;
A. Kumanogoh,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-tolerability-between-tumor-necrosis-factor-inhibitors-and-tocilizumab-for-the-treatment-of-rheumatoid-arthritis/