Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
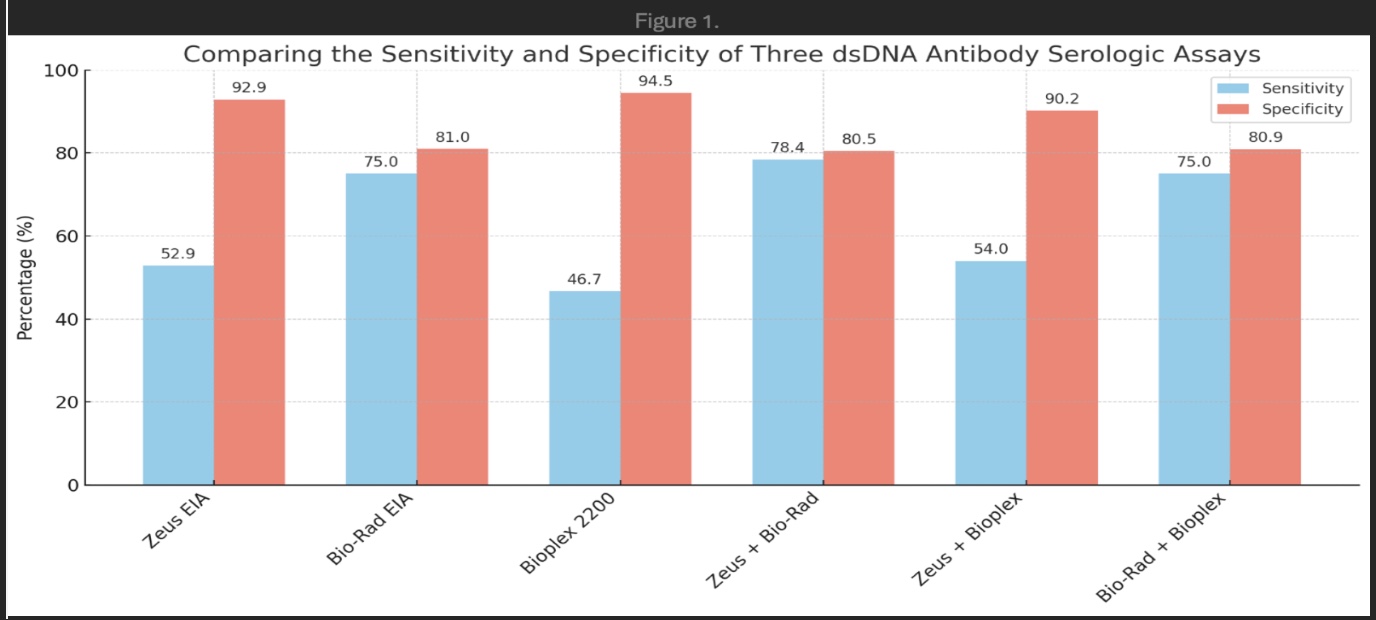
Background/Purpose: Double-stranded DNA (dsDNA) antibodies are among the most specific biomarkers for systemic lupus erythematosus (SLE). They are associated with disease activity and are incorporated into widely accepted lupus activity indices. Additionally, dsDNA antibody titers have been linked to lupus nephritis, with improvements in titers serving as important predictors of renal response. However, serologic testing for these antibodies has limitations, as antibody positivity does not always correlate with clinical SLE. Several assays are available for detecting dsDNA antibodies in serum, including the ZEUS dsDNA enzyme-linked immunosorbent semi-quantitative assay, the Bio-Rad Anti-dsDNA semi-quantitative enzyme immunoassay, and the BioPlex 2200 multiplex flow immunoassay. This study aimed to evaluate and compare the diagnostic performance of these three assays to better elucidate their clinical utility in diagnosing SLE.
Methods: A total of 78 cross-sectional serum samples from patients seen at Vanderbilt University Medical Center between November 2024 and April 2025 were analyzed using the three dsDNA antibody assays. Among these, 36 patients had a diagnosis of SLE, of whom 26 had a SLE Disease Activity Index (SLEDAI) score greater than zero (excluding points for dsDNA positivity). The remaining samples were from patients without SLE. Concordance rates between the assays were calculated, and the sensitivity and specificity of each assay, as well as combinations of assays, were assessed. Equivocal or indeterminate results were considered negative for the purposes of this analysis.
Results: The concordance rate between the Bio-Rad and BioPlex 2200 assays was 73.3%, between ZEUS and Bio-Rad was 67.6%, and between ZEUS and BioPlex 2200 was 78.5%. The Bio-Rad assay had the highest sensitivity at 75%, but the lowest specificity at 80.9%. The ZEUS and BioPlex 2200 assays demonstrated high specificity at 92.9% and 94.5%, respectively, though their sensitivity was lower at 52.9% and 46.7%. The sensitivity and specificity of a combination of these assays is depicted in Figure 1.
Conclusion: The performance of the three dsDNA antibody assays varied considerably. Concordance among the assays was suboptimal, and no single assay achieved both high sensitivity and specificity. These findings also indicate that using a combination of assays, for example through sequential testing, unfortunately does not significantly improve sensitivity or specificity of these tests. Ultimately, the results of this study suggest that existing assays, while useful adjuncts in assisting with the diagnosis of SLE, should continue to be interpreted with caution without corresponding clinical correlation given existing limitations in their diagnostic accuracy.
To cite this abstract in AMA style:
Morgado A, Rao S, Tao L, Annapureddy N. Comparison of Three Different Double-Stranded DNA Antibody Assays in the Diagnosis of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparison-of-three-different-double-stranded-dna-antibody-assays-in-the-diagnosis-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-three-different-double-stranded-dna-antibody-assays-in-the-diagnosis-of-systemic-lupus-erythematosus/