Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose : MMP-3 is an enzyme produced by synoviocytes, and is a marker of synovitis that gives a more direct indication of actual joint destruction than either C-reactive protein (CRP)in patients with rheumatoid arthritis (RA). Our aim in this study is to investigate the impact of the trend of MMP-3 and CRP during the treatment Adalimumub (ADA) and Abatacept (ABT) in the early stage of RA patients.
Methods : Among 548 patients with active RA (DAS28-CRP≥2.7) who were recruited in TBC (Tsurumai Biologics Communication) registry, 303 patients were received ADA (40mg subcutaneously every other week) and 245 patients were received ABT therapy (500mg for<60 kg; 750 mg for 60 kg-100 kg; and 1 gram for>100 kg infusion at week 0, 2, 4 and every 4 weeks). DAS28-CRP remission (DAS28-CRP<2.3) rates at 24 weeks were 34.7% (73/210) for ADA therapy and 19.4% (39/201) for ABT therapy. We analyzed 112 patients who had DAS28-CRP remission at 24 weeks, consisting of 73 patients in ADA group and 39 patients in ABT group respectively. We compared the change in serum MMP-3 and CRP levels from first administration to 24 weeks between the two groups.
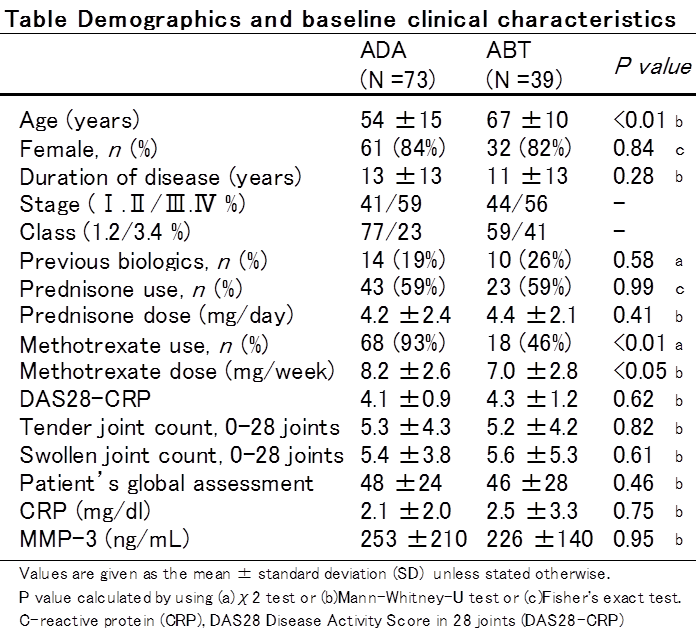
Results : While there was significant baseline difference in age, methotrexate (MTX) use and dose between the two groups, there was no significant baseline difference in serum MMP-3 and CRP levels between the two groups (Table). Serum MMP-3 and CRP levels decreased promptly at 4 weeks. The change in serum MMP-3 levels at 4, 12, and 24 weeks was greater in the ADA group than in the ABT group (Fig. 1-a). However, the change in CRP levels at 4, 12, and 24 weeks had no deference between the two groups (Fig. 1-b). The % change in serum MMP-3 levels at 12, and 24 weeks was significantly greater in the ADA group than in the ABT group (Fig. 2-a). The % change in serum CRP levels at 4 weeks was significantly greater in the ADA group than in the ABT group. However, there was no difference in the % change in CRP levels at 12, and 24 weeks between the two groups (Fig. 2-b).
Conclusion : ADA showed improvements in serum MMP-3 levels from an early stage in 24 weeks in a comparison with ABT. ADA can suppress synovitis and therefore the progression of joint destruction by strongly inhibiting MMP-3 when it is administered from an early stage.
Disclosure:
Y. Hattori,
None;
A. Kaneko,
Otsuka Pharmaceutical, Chugai Pharmaceutical, Eli Lilly and Company Japan, Santen Pharma, UCB Japan, Quintiles Transnational Japan ,
2;
D. Kida,
Mitsubishi Tanabe Pharma, Pfizer Japan, Eisai, Chugai Pharmaceutical, Abbvie,
2;
H. Ishikawa,
AstraZeneca Pharma,
2;
T. Kojima,
None;
N. Ishiguro,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken and Pfizer,
2,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken, Pfizer, Taisho-Toyama and Otsuka,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-serum-matrix-metalloproteinase-3-levels-in-rheumatoid-arthritis-after-treatment-with-adalimumub-or-abatacept-for-24-weeks/