Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Compared to healthy children, youth with juvenile myositis (JM) often report poorer health-related quality of life (HRQoL). Legacy HRQoL measures may underestimate the impact of JM due to floor/ceiling effects. Patient-Reported Outcomes Measurement Information System® (PROMIS) HRQoL measures have undergone initial validation in pediatric rheumatic diseases, including JM. PROMIS can be administered by using computerized adaptive testing (CAT) item banks or fixed short forms (FSF), but the relative benefits of CAT vs FSF are not known. The purpose of this study was to compare CAT and FSF.
Methods: JM patients (5-17 yo) and their parents were recruited at clinic visits. Demographic and clinical assessments were collected, including: Physician Global Assessment of Disease Activity (PGA); Disease Activity Score (DAS-Muscle/-Skin); muscle enzymes; and Childhood Myositis Assessment Scale (CMAS). Patients (8-17 yo) completed self-report versions and parents (of patients 5-17 yo) completed parent proxy versions of the following PROMIS domains in both CAT and FSF: Fatigue, Pain Interference, Upper Extremity Function, Mobility, Anxiety and Depressive Symptoms. To study the extreme scorers and middle scorers, participants were split into three groups by PROMIS CAT T-scores (< 45, 45-55, >55). Pearson correlations, paired t-tests, and Cohen’s d were used to compare PROMIS CAT and FSF for the entire cohort and for each of the three T-score groupings.
Results: Data from 67 patient-parent dyads were analyzed. Most patients were 8-17 yo (n=49; 73%), diagnosed with juvenile dermatomyositis (n=61; 91%), female (n=56; 84%), and white (n=51; 76%). Median [IQR] age of onset was 5.2 [3.9, 7.1] and age at initial study visit was 11.8 [7.4, 15.2]. Median [IQR] clinical data were: PGA 0.5 [0.0, 1.5]; DAS-Muscle 0.0 [0.0, 1.0]; DAS-Skin 1.0 [0.0, 5.0]; and CMAS 52.0 [50.0, 52.0]. Median [IQR] muscle enzyme values were: CPK 92.0 [69.0, 130.0]; AST 28.5 [23.0, 32.8]; ALT 14.0 [11.2, 17.8]; LDH 257.0 [223.0, 307.0]; and Aldolase 5.5 [4.8, 6.0]. PROMIS CAT and FSF were highly correlated (Pearson’s correlation coefficients ranging 0.79-0.92) (Table 1). Mean scores between CAT and FSF were not significantly different except in parent proxy anxiety and fatigue, though effect sizes were modest (0.508 and 0.317, respectively). Correlations between CAT and FSF varied based on domains, patient vs parent report, and T-score groupings (Table 2). Review of scatterplots showed floor/ceiling effect at the less symptomatic extreme in all FSF domains (Figure 1).
Conclusion: PROMIS CAT is feasible in clinical settings and is comparable to FSF. Additionally, CAT had less pronounced floor/ceiling effects than FSF, allowing detection of individual differences in low symptom/disability scorers. CAT is recommended for long-term follow-up of JM patients given that deconditioning often persists even in remission. Future studies should focus on multicenter replication of these findings, benefits of CAT vs FSF in patients with more severe symptoms, and clinical interpretability (e.g. minimal important differences, severity strata).
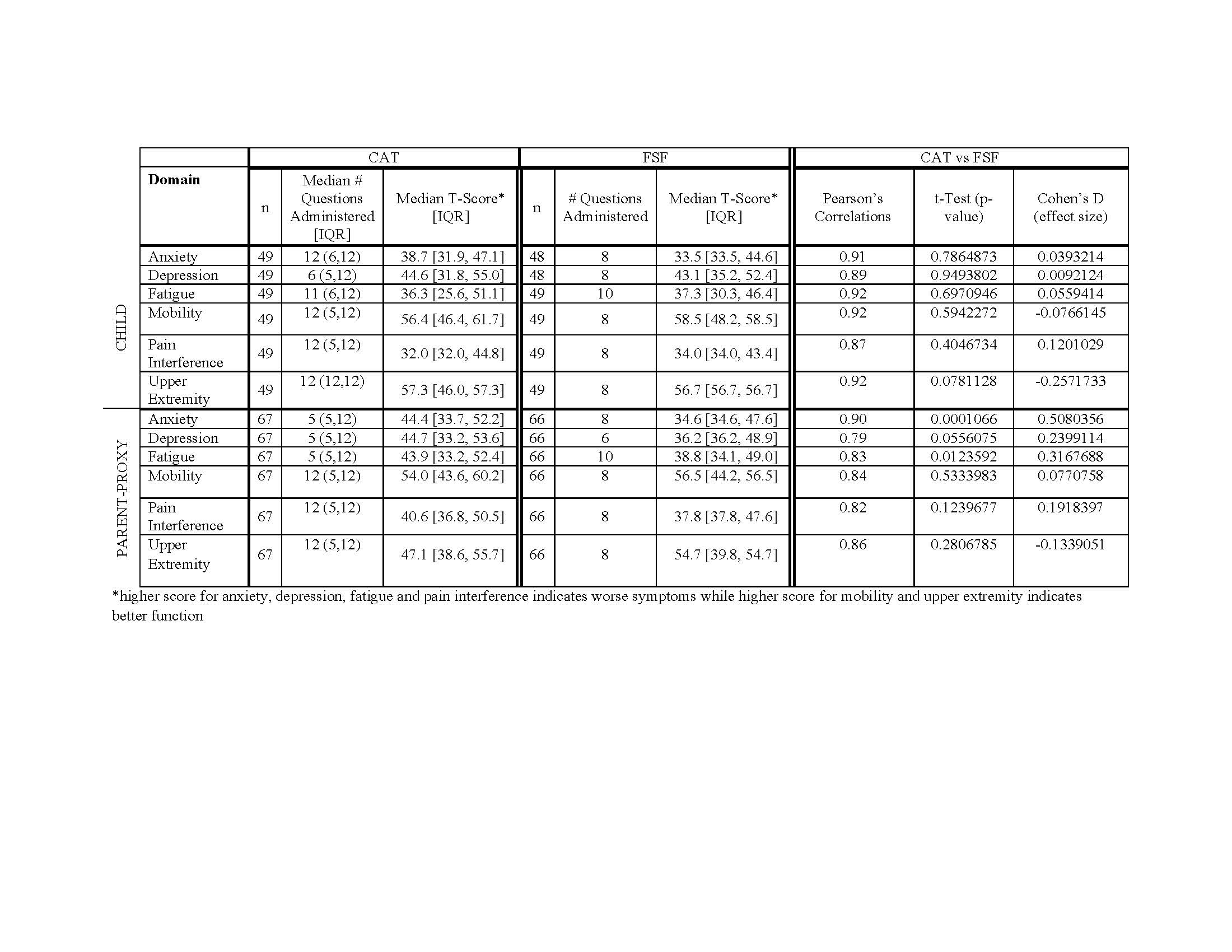
PROMISCATvsFSF-JM-Abstract-FINAL-2019-06-02-TABLE-1-ONLY
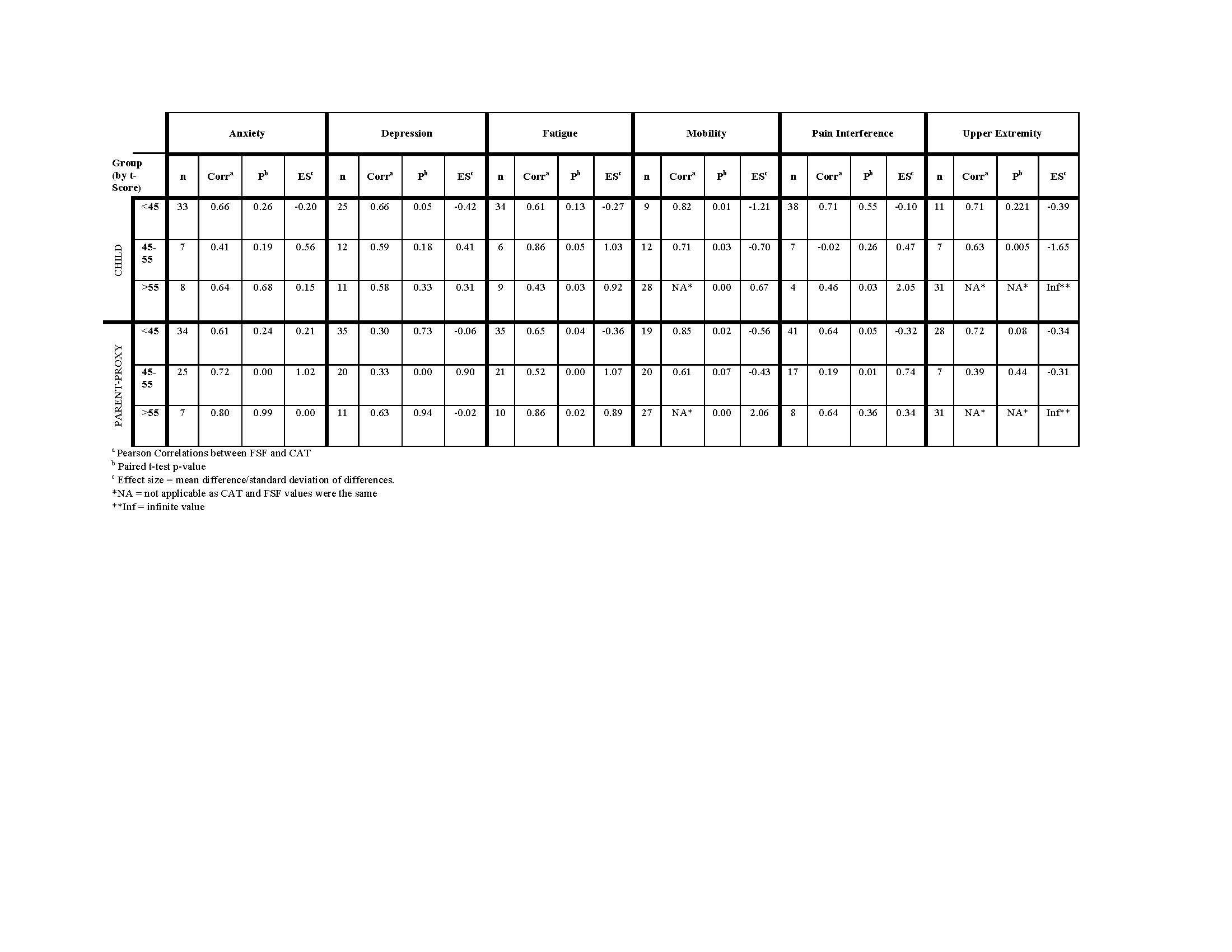
PROMISCATvsFSF-JM-Abstract-FINAL-2019-06-02-TABLE-2-ONLY
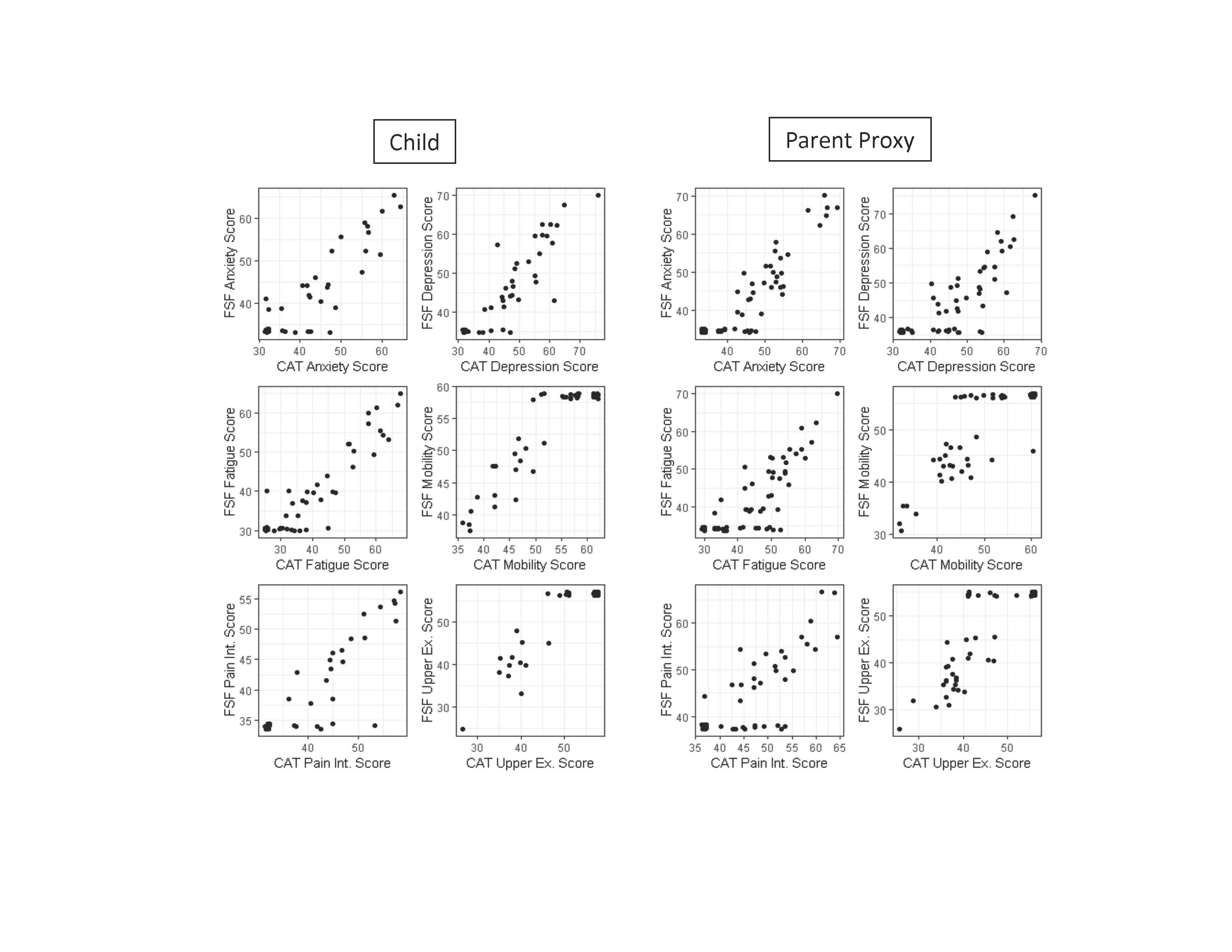
PROMISCATvsFSF-JM-Abstract-FINAL-2019-06-02-FIGURE-1-ONLY
To cite this abstract in AMA style:
Patel R, Esparza V, Lai J, Gray E, Chang R, Cella D, Ardalan K. Comparison of PROMIS Computerized Adaptive Testing-Administered Item Banks versus Fixed Short Forms in Juvenile Myositis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-of-promis-computerized-adaptive-testing-administered-item-banks-versus-fixed-short-forms-in-juvenile-myositis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-promis-computerized-adaptive-testing-administered-item-banks-versus-fixed-short-forms-in-juvenile-myositis/
