Session Information
Date: Sunday, November 5, 2017
Title: ARHP Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Denosumab (Dmab) is an antiresorptive agent with an approximate half-life of 26 days and according to the prescribing information the recommended dose is 60 mg subcutaneous every 6 months. Although in real world clinical practice, strict adherence to this dosing regimen is frequently hampered due to delays in prior authorizations, follow up appointment scheduling and missed visits. We hypothesized that patients who did not adhere to the standard dosing regimen would have impaired efficacy of Dmab. The objective of this study is to determine whether there is difference in bone mineral density (BMD) after 2 years of therapy in patients who received Dmab at recommended 6 month intervals as compared with patients who did not.
Methods: All patients between 2009 and 2015 with a primary diagnosis of post-menopausal osteoporosis that had received 4 doses of Dmab in 2 consecutive years were identified from the electronic data base. Furthermore patients were required to have a baseline DXA within 2 years prior to Dmab initiation and a follow up DXA within 2-3 years thereafter done on the same GE Lunar machine (least significant change for hip 0.036 g/cm2). Patient records were reviewed to obtain information on demographics, weight, smoking history, baseline vitamin D level, Dmab administration and DXA results. Change in BMD at lowest T-score of hip or femoral neck for each patient was used as the outcome measure. Adherence to therapy was defined as receiving subsequent injections at 6 month ± 4 week intervals. Patients were divided into 3 groups with Group 1 being adherent with all injections. Patients in Group 2 were adherent with 2 of 3 subsequent injections whereas Group 3 patients received subsequent injections at 8-12 month intervals and were considered to be non-adherent. Descriptive analysis included continuous variables (means ± SD) and categorical variables (%). Data were compared by using ANOVA.
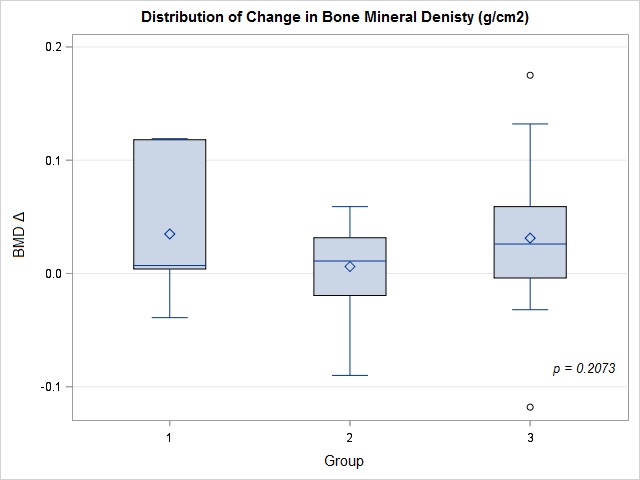
Results: 50 patients, all females, with mean age of 75 yrs (± 9.4), 74% were Caucasian, 12% African American, 6% Hispanic and 8% were of other ethnicities. 60% smokers and 56% had history of fragility fractures. Mean Vitamin D level was 39.3 ng/ml (± 21.5). The mean lowest BMD at the hip or femoral neck at baseline and follow up were 0.705 g/cm2 (± 0.130) and 0.726 g/cm2 (± 0.130), respectively. 14% of patients were adherent with all 3 injections whereas 40% were categorized as Group 2. 46% were non adherent. The mean change in BMD stratified by groups was 0.035 g/cm2 (± 0.061) for Group 1, 0.006 g/cm2 (± 0.035) for Group 2 and 0.031 g/cm2 (± 0.057) for Group 3. There was no significant difference in mean lowest BMD change at the hip or femoral neck between the 3 groups through ANOVA analysis (p=0.2073).
Conclusion: Despite low adherence to the standard dosing regimen for Dmab there was no significant difference in change of BMD in patients who were not adherent to the current prescribing information.
To cite this abstract in AMA style:
Syed NA, Wiqar M, Einstadter D, Magrey MN. Comparison of Outcomes in Osteoporosis in Patients on Denosumab between Standard and Non-Standard Dosing Intervals [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparison-of-outcomes-in-osteoporosis-in-patients-on-denosumab-between-standard-and-non-standard-dosing-intervals/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-outcomes-in-osteoporosis-in-patients-on-denosumab-between-standard-and-non-standard-dosing-intervals/