Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Guselkumab, a fully human IL-23p19-subunit inhibitor, has demonstrated significant and durable efficacy and high rates of patient retention in randomized controlled trials of adults with active psoriatic arthritis (PsA).1,2 A recent analysis of US health plan claims data showed persistence of on-label guselkumab (i.e., following US FDA-approved dosing of 100 mg administered by subcutaneous [SC] injection at Week [W] 0, W4, and every 8 weeks) at 12 months to be ~3x that of a first SC tumor necrosis factor inhibitor (TNFi).3 Evidence in real-world adults with active PsA is also needed to compare on-label treatment persistence between patients who initiate guselkumab vs a first SC IL-17A inhibitor (IL-17Ai).
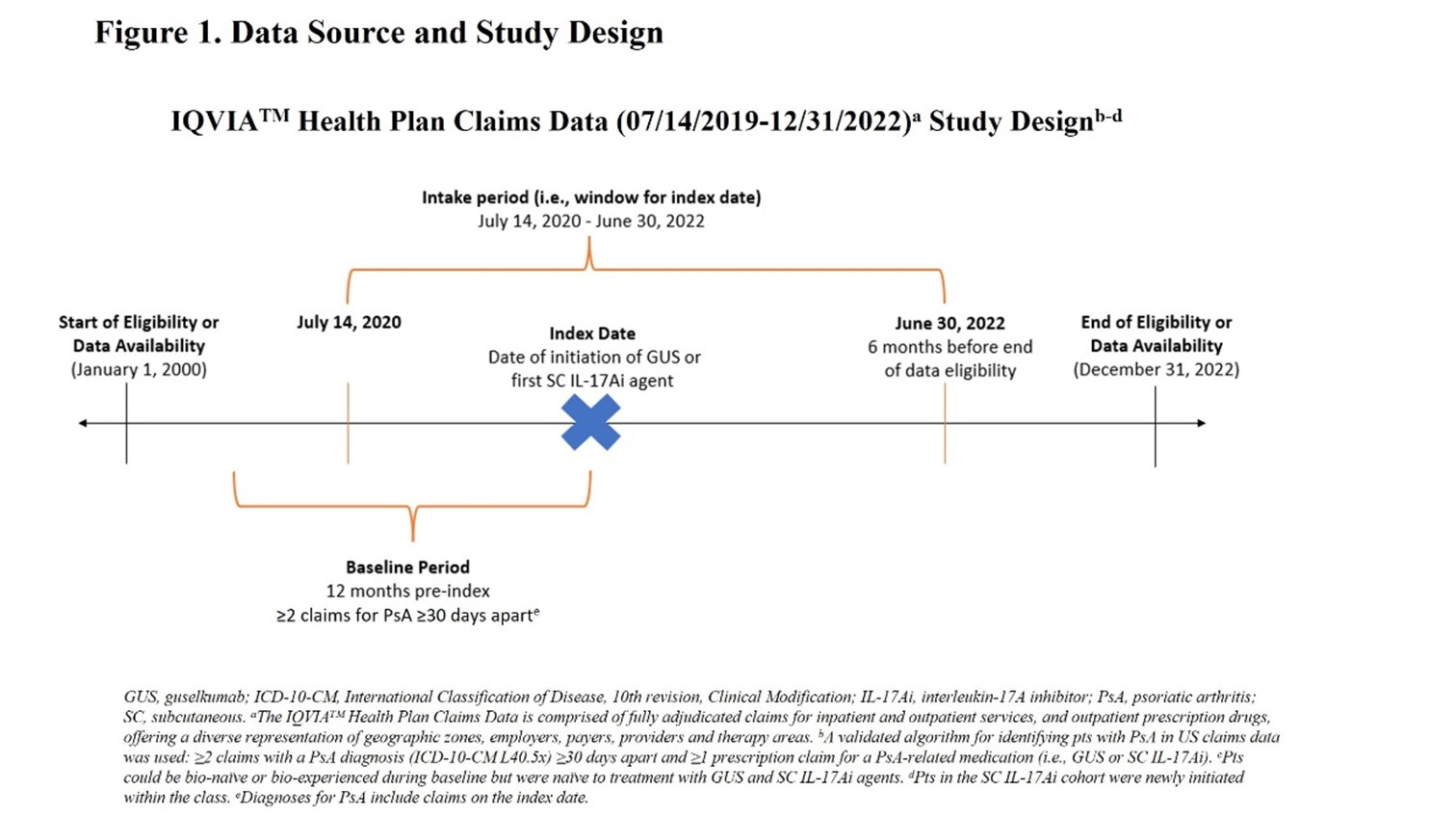
Methods: Biologic-naïve and biologic-experienced adults with active PsA, defined by ≥2 claims with a PsA diagnosis (ICD-10-CM L40.5x) ≥30 days apart within 12 consecutive months before or on the index date (the first claim/start of treatment), and ≥1 claim for guselkumab or first SC IL-17Ai (ixekizumab or secukinumab) between July 14, 2020 and June 30, 2022 were identified in IQVIA PharMetrics® Plus. Selected patients also had ≥12 months of continuous enrollment and no claim for a potentially confounding rheumatic disease within 12 months preceding the index date (Figure 1). Baseline patient characteristics were balanced between the guselkumab and SC IL-17Ai cohorts using propensity score (standardized mortality ratio [SMR]) weighting. On-label persistence (absence of discontinuation or dose escalation/reduction relative to FDA prescribing information) was described and compared using Kaplan-Meier survival analysis and Cox proportional hazard models in the weighted treatment cohorts.
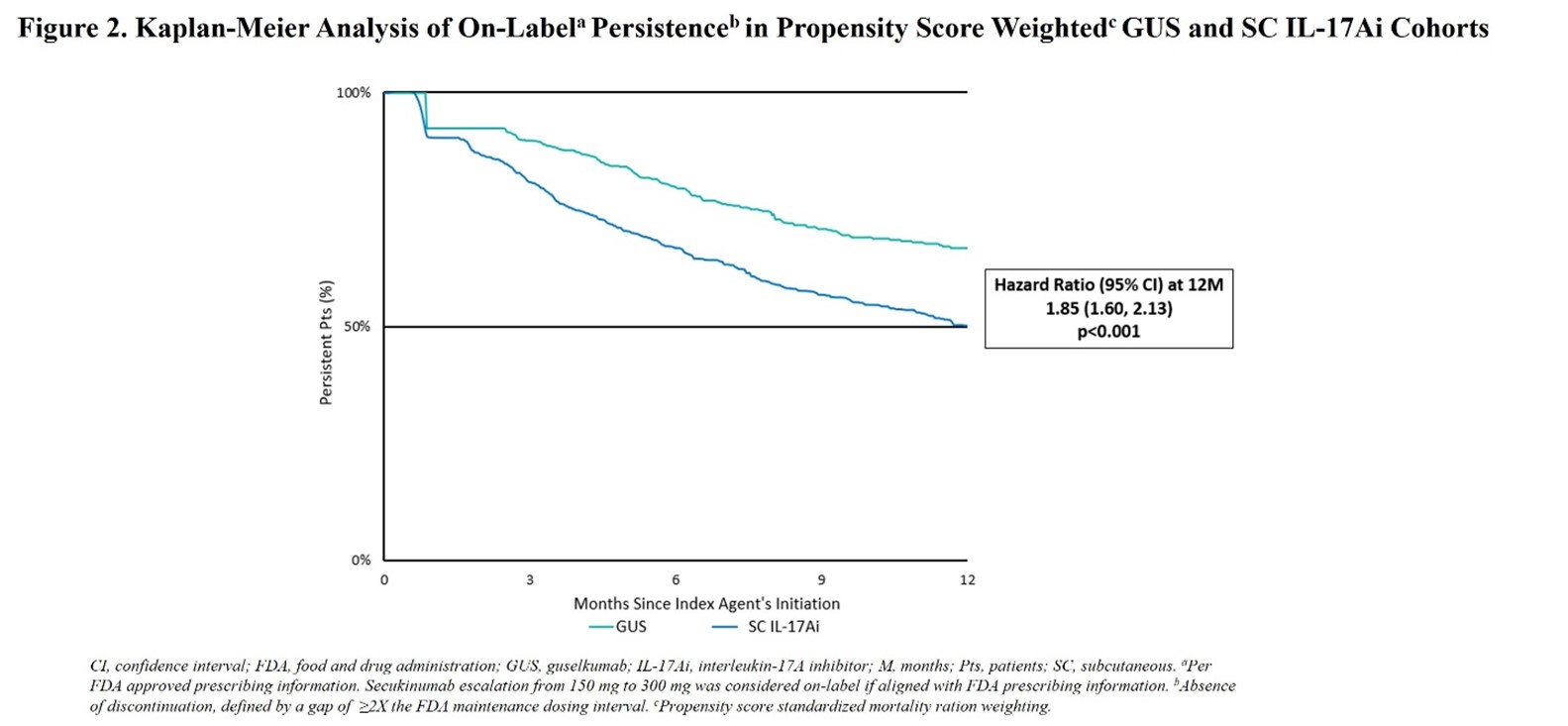
Results: The guselkumab and SC IL-17Ai cohorts included 910 (mean age 50 years, 60% female) and 2,743 (50 years, 58% female) patients, respectively. After weighting, baseline characteristics were well-balanced, with mean follow-up of 13.5-13.7 months and 52% of patients across cohorts having received biologics in the previous 12 months. Respective rates of treatment persistence at 3, 6, 9, and 12 months were 90%, 80%, 71%, and 67% for guselkumab vs 81%, 67%, 57%, and 50% for SC IL-17Ai (overall log-rank p< 0.001). Patients in the guselkumab cohort were significantly more likely than those in the SC IL-17Ai cohort to remain persistent on treatment at each timepoint assessed, with increasing hazard ratios (HRs) from 3 to 12 (HR=1.85; p< 0.001) months (Figure 2). Median time to discontinuation was not reached for guselkumab and was 12.3 months for SC IL-17Ai.
Conclusion: In this real-world study employing primarily commercial US health plan claims data to assess on-label treatment persistence in patients with PsA, guselkumab was associated with a significantly greater (nearly 2x) likelihood of persistence at 12 months compared with an initial SC IL-17Ai.
References:
1. Ritchlin CT. RMD Open. 2021; 7:e001457.
2. McInnes IB. Arthritis Rheumatol. 2022; 74: 475-85.
3. Walsh J. Comparison of On-label Treatment Persistence in Real-world Patents With PsA Receiving Guselkumab Versus Subcutaneous TNF Inhibitors. Poster presented at: CCR-W 2023; San Diego, CA.
To cite this abstract in AMA style:
Mease P, Zhao R, Ferrante S, Shiff N, Srinivas P, Chakravarty S, Walsh J. Comparison of On-Label Treatment Persistence in Real-World Patients with Psoriatic Arthritis Receiving Guselkumab versus Subcutaneous IL-17A Inhibitors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparison-of-on-label-treatment-persistence-in-real-world-patients-with-psoriatic-arthritis-receiving-guselkumab-versus-subcutaneous-il-17a-inhibitors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-on-label-treatment-persistence-in-real-world-patients-with-psoriatic-arthritis-receiving-guselkumab-versus-subcutaneous-il-17a-inhibitors/