Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Mizoribine (MZR) is an immunosuppressant working as an inhibitor of purine synthesis, which mechanism of action is similar to mycophenolate mofetil. MZR is approved as the treatment of rheumatoid arthritis and lupus nephritis in Japan, and is reported to have effect in ANCA-associated renal vasculitis. But the data about the effect in remission maintenance and glucocorticoid dose reduction in patients with ANCA-associated vasculitis (AAV) are limited.
Methods: We retrospectively reviewed charts of patients with AAV who used MZR or azathioprine(AZA) at St. Luke’s International Hospital, Tokyo, Japan from January 2004 to December 2018. We investigated basic demographics, types of AAV, results of blood tests, and medications used in the treatment of AAV including glucocorticoid and other immunosuppressants(IS). We defined flare of AAV as a new onset AAV-related symptom that required increase or initiation of glucocorticoids or IS. We calculated and flare-free rate and compare between MZR treatment group and AZA treatment group with Kaplan Meier curve by using log rank test. We also check the dose reduction effect in glucocorticoids before and after MZR treatment by using Wilcoxon signed-rank test.
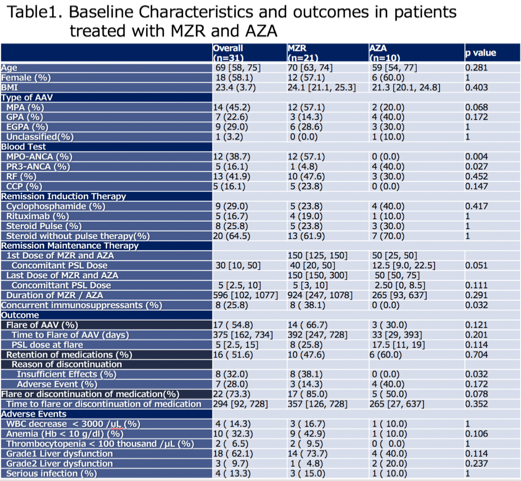
Results: We identified 31 patients in total, and there were 21 and 10 patients who were primarily treated with MZR and AZA. Baseline characteristics are shown in Table1. 8 Patents (38.1%) in MZR-treatment group used concurrent IS (5: methotrexate, 1: azathioprine, 1: abatacept and 1: tacrolimus). Flare-up of AAV was seen in 14 (66.7%) in MZR and 3 (30.0%) in AZA-treated group, but there was no significant differences in the flare free rate (Figure 1). Dose of glucocorticoids significantly decreased after using MZR and steroid dose at flare is low (median 40mg [20, 50] vs 5 mg [3, 10]; p < 0.01, Wilcoxon signed-rank test). Mild liver dysfunction is common in MZR group, but just 3 (14.3%) patients discontinued MZR for adverse events(AE) (2: allergy, 1: infection) and major reason for discontinuation was insufficient effect, although 40% of patients treated with AZA had to discontinue due to bone marrow suppression and severe liver dysfunctions.
Conclusion: MZR seemed to have significant steroid-sparing effect in patients with AAV although rate of flare-up is high. Given the safe profile of MZR, MZR may be a useful agent can be used as alternative in patients who cannot continue AZA or other IS for AE, or in addition to regular IS for AAV.
To cite this abstract in AMA style:
Fukui S, Kawaai S, Kidoguchi G, Nakai T, Ozawa H, Koido A, Ikeda Y, Suda M, Yanaoka H, Shimizu H, Tamaki H, Tsuda T, Kishimoto M, Yamaguchi K, Okada M. Comparison of Mizoribine with Azathioprine in Efficacy and Safety for ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-of-mizoribine-with-azathioprine-in-efficacy-and-safety-for-anca-associated-vasculitis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-mizoribine-with-azathioprine-in-efficacy-and-safety-for-anca-associated-vasculitis/