Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: B cells play an important role in the pathogenesis of systemic lupus erythematosus(SLE).Multiple biological agents with B lymphocyte as the therapeutic target have recently emerged as potential novel treatments for SLE. This study aimed to compare the efficacy and safety of B lymphocyte stimulation inhibitor ,belimumab and telitacicept, in the treatment of SLE under standard treatment in real-world clinical practice.
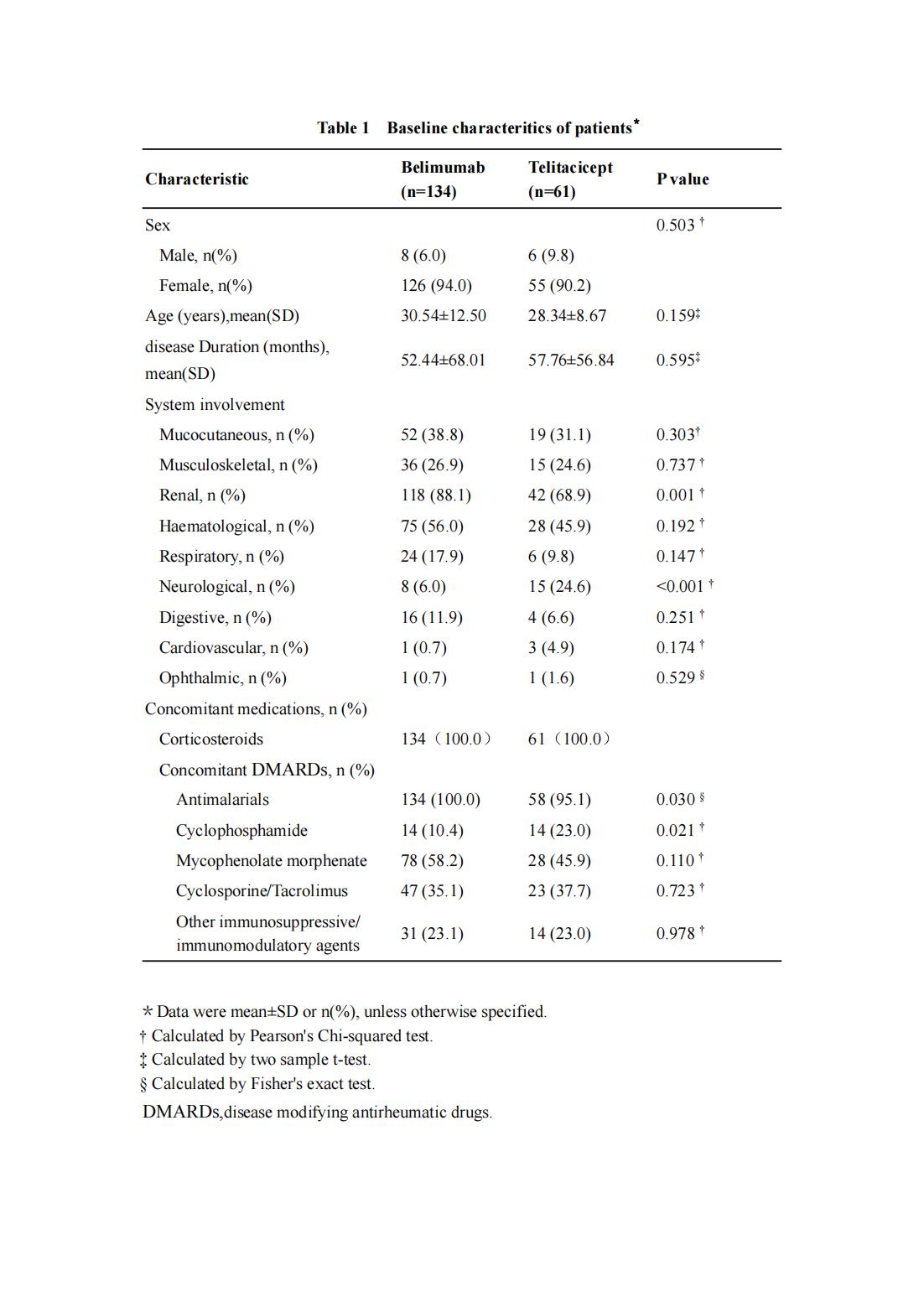
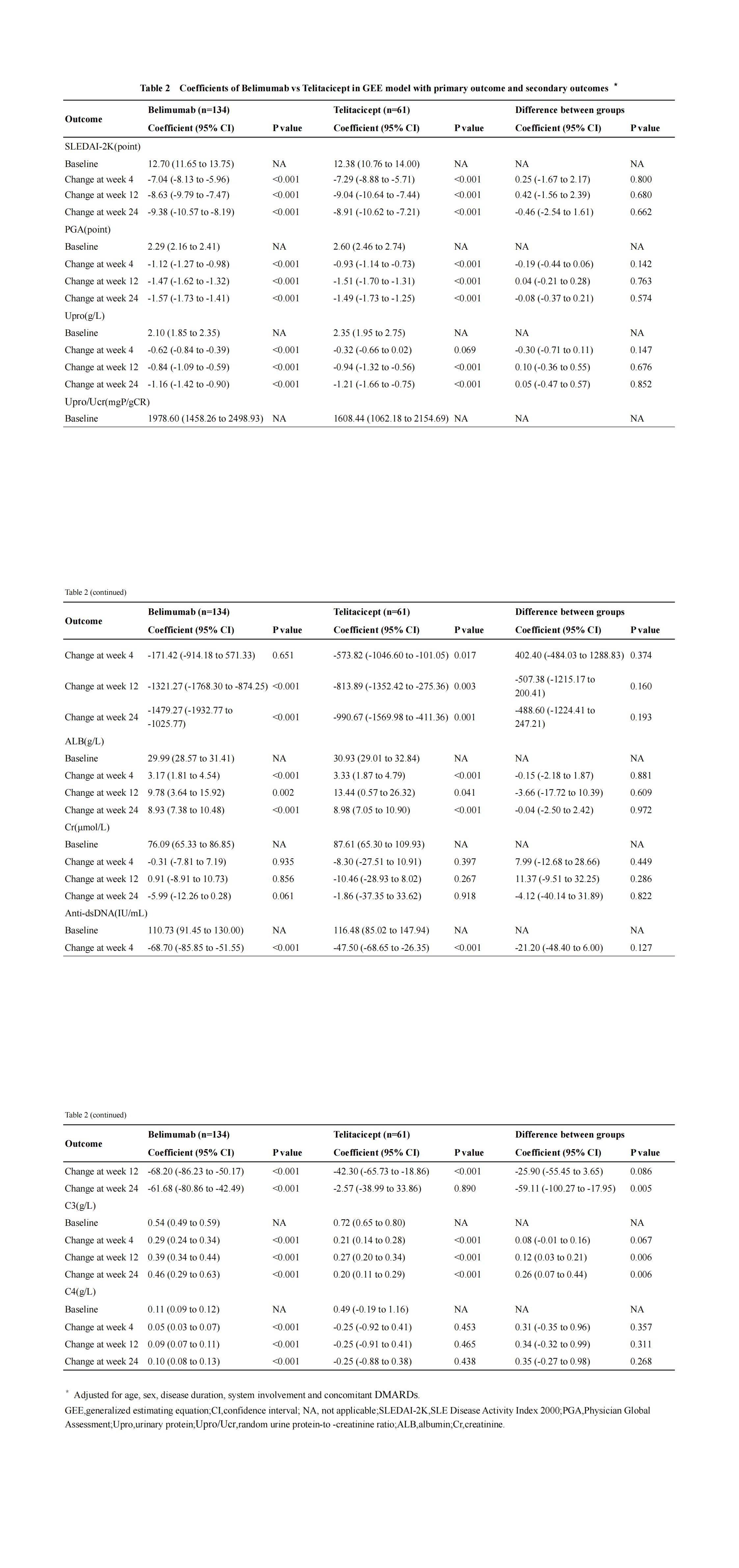
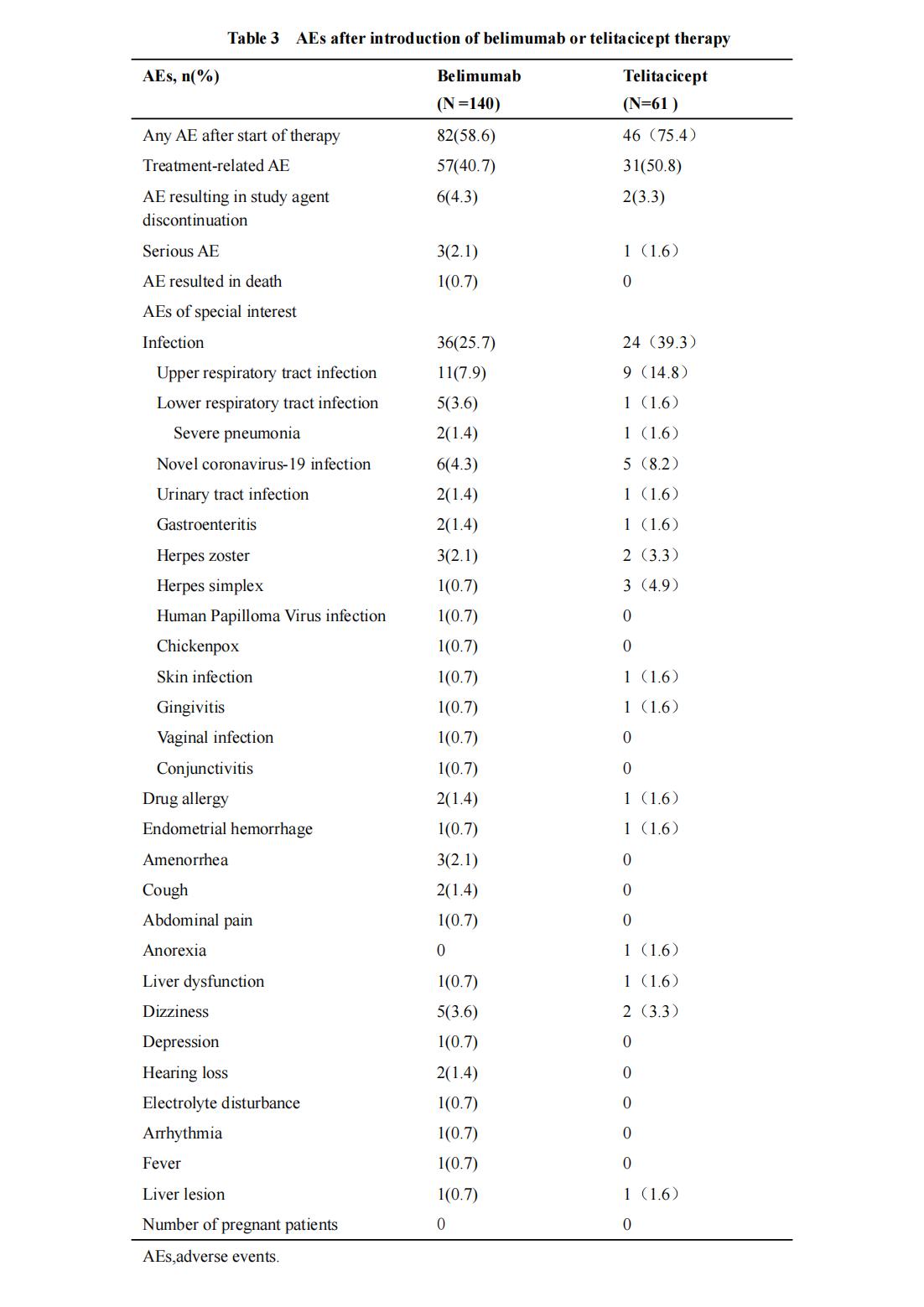
Methods: SLE patients treated with belimumab or telitacicept at the Rheumatology and Immunology Department of the First Affiliated Hospital of Guangxi Medical University from April 1, 2020 to December 31, 2023 were collected. The primary outcomes were changes in SLEDAI-2K and PGA scores and were observed at week 4, 12 and 24 after treatment. Secondary outcomes included urinary protein(Upro), random urine protein-to-creatinine ratio(Upro/Ucr),albumin, creatinine,complement C3 and C4, anti-dsDNA and adverse events(AEs).
Results: There were a total of 195 patients, including 134 in belimumab group, with an average age of 30.54±12.50 years,disease duration 52.44±68.01 months,126 (94.0%) were female.61 in telitacicept group with an average age of 28.34±8.67 years,disease duration 57.76±56.84 months,55 (90.2%) were female.After treatment, the level of anti-dsDNA and complement C3 in belimumab group decreased more obviously than that in telitacicept group, and the decrease of anti-dsDNA at week 24, increase of complement C3 at week 12 and week 24 were statistically significant between two groups. There was no statistical difference in the improvement of Upro,Upro/Ucr,complement C4 between two groups after treatment.The overall AE rates were 58.6% and 75.4% in the belimumab group and telitacicept group, respectively,including infection (25.7% vs 39.4%). One death due to cerebral hemorrhage,two severe pneumonia and one depression in belimumab group.More upper respiratory tract infections and viral infections in telitacicept group.
Conclusion: Belimumab or telitacicept combined with standard treatment significantly improved disease activity in SLE patients but with no difference in efficacy.The incidence of infection with belimumab is relatively low, and the upper respiratory tract infection of telitacicept is more common.
To cite this abstract in AMA style:
wei w, lei l, Zhao C, mo h, chen z, zheng l, wen j, Qin F, liao x, zeng w, dong f. Comparison of Efficacy and Safety Between Belimumab and Telitacicept in the Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparison-of-efficacy-and-safety-between-belimumab-and-telitacicept-in-the-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-efficacy-and-safety-between-belimumab-and-telitacicept-in-the-treatment-of-systemic-lupus-erythematosus/