Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
The individual response to anti-TNFs etanercept (ETA), infliximab
(INF)
and adalimumab (ADA) in rheumatoid arthritis (RA) may vary. Options for
managing inadequate response include dose escalation of the anti-TNF agent and/or
intensification of co-therapy, which can lead to higher drug costs, patient
inconvenience and greater risk of adverse events. The Canadian
Drug Utilization in Rheumatoid Arthritis (CADURA) study compared dose
escalation,
intensification of DMARDS and steroids, and switches to another
biologic between patients with RA initiating ETA, ADA or INF.
Methods:
This was a retrospective chart review of RA patients treated in 11 clinical
practices across Canada. Patients were 18 years or older who initiated an anti-TNF
between January 1, 2006 and December 31, 2012, with no prior use of biologic
DMARD. Outcomes were assessed over 12 and 18 months. For the 12- month
analysis, patients were required to have ≥3 physician visits following
initiation and ≥1 visit during months 9-15; the 18 month analysis also required
≥ 1 visit during months 15-21. Dose-escalation of anti-TNF was defined as the
first occurrence of any upward adjustment in dose or dosing frequency of the
anti-TNF from the label indication: ETA, 25 mg twice weekly or 50 mg once
weekly; ADA, 40 mg once every other week; INF, 3mg/kg every 8 weeks after the
3rd infusion. Additional outcomes were: 1) anti-TNF dose-escalation and/or
DMARD intensification and 2) anti-TNF dose-escalation and/or DMARD and/or
steroid intensification. Intensification included any increase in dose or dosing
frequency, any addition, any switch from oral to subcutaneous injection
(DMARD), or any intramuscular or intra-articular injections (steroid) 3 months after
initiation of the anti-TNF.
Results:
The 12-month analysis included 314 patients (mean age 56.3 years [range: 21-90
yrs]; 77% female; mean RA duration 9.0 yrs [range: 2-26 yrs]). Among patients
initiating ADA, 83% had concomitant DMARD vs 72% of patients
initiating ETA
and 75% initiating INF.
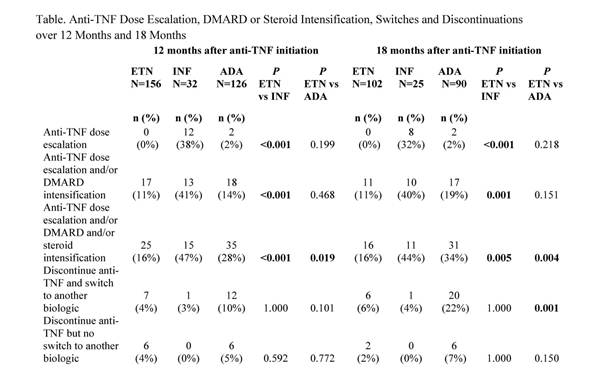
There were 217 patients in the 18-month analysis. No dose escalation occurred with
ETN over 12 and 18 months, vs 38% and 32% for INF (p<0.001) and 2% and 2%
for ADA (p=0.199, p=0.218). Over 18 months, dose escalation and/or DMARD and/or
steroid intensification was less frequent among ETA (16%) vs INF (44%, p=0.005)
and ADA (34%, p=0.004). By 18 months, 22% of patients initiating ADA had
switched to another biologic compared with 6% of ETN patients (p=0.001).
Conclusion:
Physicians more frequently used dose escalation when treating with INF, and
DMARD and/or steroid intensification when treating with ADA. Patients treated
with ETN had no dose escalation, were less likely to have DMARD and/or steroid
intensification than patients initiating INF or ADA over 12 and 18 month, and
were less likely to switch to another biologic over 18 months than patients
initiating ADA.
To cite this abstract in AMA style:
Thorne JC, Boire G, Chow A, Garces K, Liu F, Poulin-Costello M, Walker V, Haraoui B. Comparison of Dose Escalation and Co-Therapy Intensification Between Patients with Rheumatoid Arthritis Initiating Biologic Treatment with Etanercept, Adalimumab and Infliximab [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/comparison-of-dose-escalation-and-co-therapy-intensification-between-patients-with-rheumatoid-arthritis-initiating-biologic-treatment-with-etanercept-adalimumab-and-infliximab/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-dose-escalation-and-co-therapy-intensification-between-patients-with-rheumatoid-arthritis-initiating-biologic-treatment-with-etanercept-adalimumab-and-infliximab/