Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Since discontinuation may be a surrogate for ineffectiveness, we measured rates and reasons for biologic discontinuation in a real-world setting.
Methods: From 1998 to 2011, all medication use was measured every 6 months via questionnaire in a US-wide longitudinal study of RA patients. We limited our analysis to patients with a baseline observation immediately before initiating their first (1st) or second different (2nd) biologic and at least one observation after initiation. Patients reported their primary reason for discontinuation. Time-on-drug survival analyses were conducted for individual biologics and groups and annual rates reported. Survival of anti-TNFs and non-anti-TNFs was compared, in crude and adjusted in propensity score analyses.
Results: A total of 2,340 RA patients initiated their 1st biologic; 1,148 (49%) discontinued and 1,128 initiated their 2nd; 567 (50%) discontinued. The vast majority initiated one of the following: etanercept (1st 44%, 28% 2nd), infliximab (1st 37%, 38% 2nd), and adalimumab (1st 13%, 19% 2nd). The annual discontinuation rate of all 1st biologics was 18% (95% CI 17-19%) and 2nd was 21% (19-23%). Annual discontinuation rates for 1st and 2nd by drug was 15% (14-16%) and 16% (14-19%) for etanercept; 19% (17-21%) and 18% (16-20%) for infliximab; and 20% (17-23%) and 26% (22-32%) for adalimumab. Anakinra had the highest annual rates, 1st 59% (44-78%) and 2nd 106% (74-151%). Patients who started a biologic after 2005 had higher annual discontinuation rates, 1st 25% (22-29%) and 2nd 31% (27-35%). Anti-TNF had lower annual discontinuation rates vs non-anti-TNF (1st); crude HR 0.48 (0.34-0.69) and after propensity score adjustment, HR of 0.60 (0.40-0.90). For 2nd line, a crude HR of 0.64 (0.48-0.84) and adjusted HR of 0.81 (0.59-1.11) (see Figures). Reporting a side effect had the highest discontinuation rate, 1st 63% and 2nd =81%, followed by reporting the drug was “not working”, 1st 49% and 2nd 60%.
Conclusion: In this large cohort, RA patients tend to remain on their initial and second biologic for relatively long periods suggesting the drugs’ effectiveness. Discontinuation rates were higher for non-anti-TNFs and in patients who initiated a biologic more recently when more treatment options were available. Contrary to recent findings, there was no statistically significant difference in discontinuation rates of anti-TNFs and non-anti TNFs as second biologic treatment. 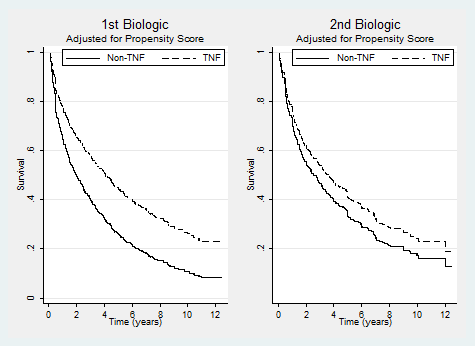
Disclosure:
S. Ramiro,
None;
F. Wolfe,
None;
D. J. Harrison,
Amgen,
1,
Amgen,
3;
G. Joseph,
Amgen Inc.,
1,
Amgen Inc.,
3;
D. H. Collier,
Amgen Inc.,
1,
Amgen Inc.,
3;
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer Inc., Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
5,
Imaging Rheumatology,
4;
R. Landewé,
Rheumatology Consultancy BV ,
4,
Abbott, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB, Wyeth,
5;
K. Michaud,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-discontinuation-rates-by-biologic-since-1998-in-us-patients-with-rheumatoid-arthritis/
