Session Information
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Head-to-head comparisons of biological (b)DMARDs in the treatment of RA should provide rigorous evidence on the comparative efficacy of different treatments. Although there are several head-to-head trials comparing TNF inhibitors (TNFi) with bDMARDs that have different mechanisms of action,1-3 there have been no reports of prospective head-to-head trials comparing the efficacy and safety of bDMARDS within the same class, including TNFi.
Methods: EXXELERATE (NCT01500278) was a 104-wk randomized, investigator-blind, parallel-group, head-to-head superiority study comparing the early (Wk 12)- and later (Wk 104)-term efficacy and safety of certolizumab pegol (CZP)+MTX and adalimumab (ADA)+MTX (Figure). Patients (Pts) were randomized 1:1 to CZP+MTX or ADA+MTX. At Wk 12, pts were classified as responders (achieving either DAS28[ESR] ≤3.2 or DAS28[ESR] reduction from baseline [BL] of ≥1.2 at Wk 12) or non-responders (NR). NRs to one were switched to the respective other TNFi (Figure). Primary endpoints were the percentage of pts achieving ACR20 at Wk 12 and low disease activity (LDA; DAS28[ESR] ≤3.2) at Wk 104 (Wk 12 NRs were considered LDA NRs). Secondary endpoints included the proportion of pts achieving ACR20 at Wk 6, DAS28(ESR) LDA at Wks 6, 12, 52, and the proportion of Wk 12 responding pts achieving LDA at Wk 104. Exploratory endpoints included the proportion of Wk 12 responding pts achieving ACR20/50/70, and DAS28(ESR) and CDAI defined LDA and remission (REM), at each study visit.
Results: At BL, 915 pts were randomized to either CZP+MTX (n=457) or ADA+MTX (n=458). At Wk 12 there were 359 CZP+MTX (78.6%) and 369 ADA+MTX (80.6%) responder pts. No statistically significant difference was observed between ACR20 response at Wk 12 (69.2% and 71.4%; odds ratio (OR): 0.90 [95% CI: 0.67, 1.20]), and DAS28(ESR) LDA at Wk 104 (35.5% and 33.5%; OR: 1.09 [95% CI: 0.82, 1.45]) for CZP+MTX and ADA+MTX, respectively. No differences between treatment arms were evident in secondary and exploratory efficacy endpoints (Table). A similar proportion of CZP+MTX and ADA+MTX pts reported treatment emergent adverse events (TEAEs; 75.4% and 73.8%), serious TEAEs (13.0% and 11.1%), and serious infections and infestations (3.3% and 3.1%), by treatment at AE onset (event rate per 100 pt years 257.5 vs 260.0).
Conclusion: EXXELERATE, the first direct head-to-head comparison of two TNFis, reinforces the early and later term efficacy of both CZP and ADA in combination with MTX without demonstrating clinical evidence of differences between both agents. CZP+MTX and ADA+MTX demonstrated comparable safety over 2 years. References: 1. Porter D. Lancet 2016;S0140-6736(16)00380–9; 2. Gabay C. Lancet 2013;381(9877):1541–1550; 3. Weinblatt M. Arthritis Rheum 2013;65(1):28–38 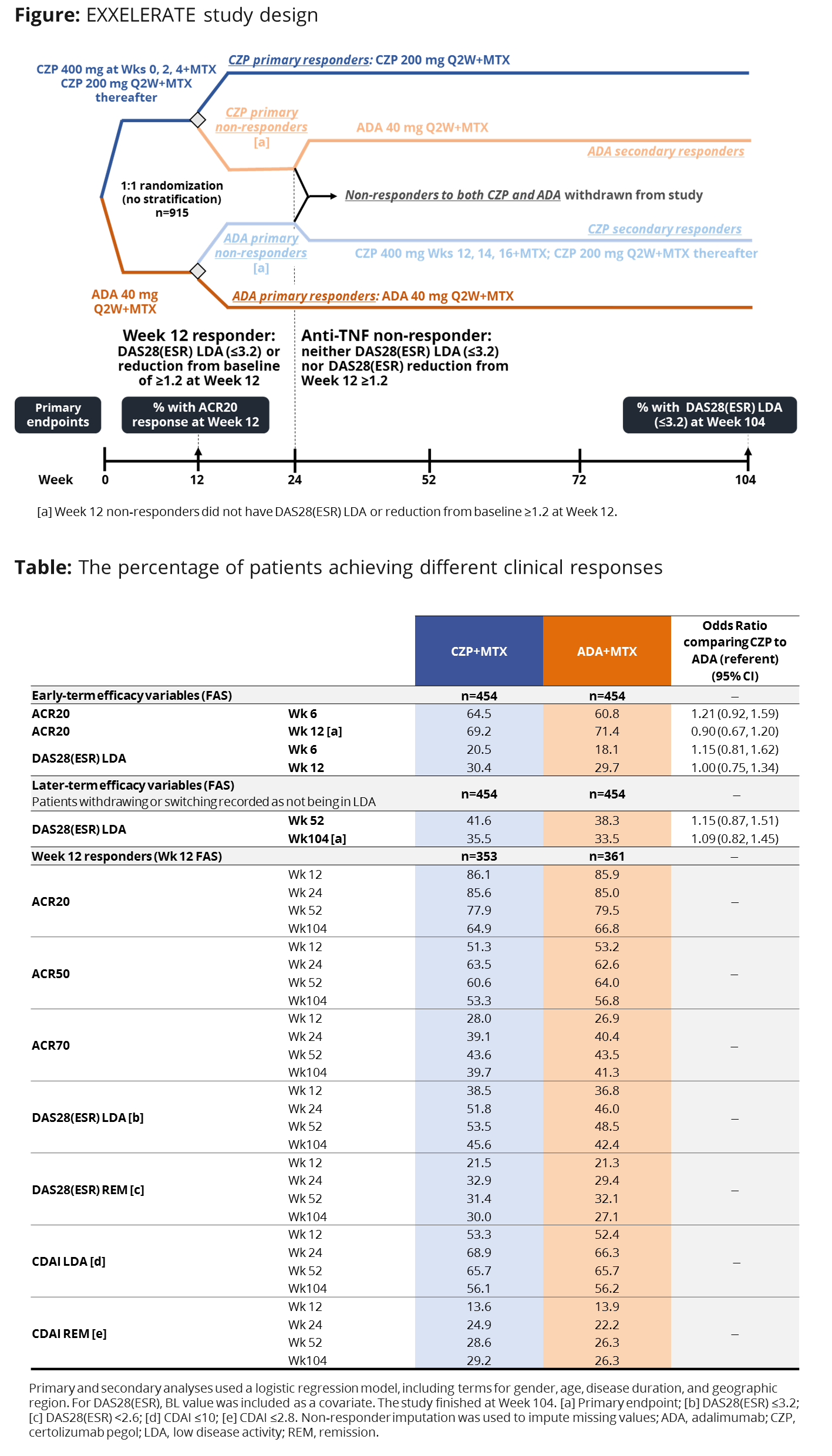
To cite this abstract in AMA style:
Fleischmann R, Burmester GR, Combe B, Curtis JR, Hall S, Haraoui B, van Vollenhoven R, Cioffi C, Ecoffet C, Ionescu L, Gervitz L, Peterson L, Smolen J. Comparison of Certolizumab Pegol Versus Adalimumab: 2 Year Efficacy and Safety Results from a Superiority, Investigator-Blind, Head-to-Head Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/comparison-of-certolizumab-pegol-versus-adalimumab-2-year-efficacy-and-safety-results-from-a-superiority-investigator-blind-head-to-head-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-certolizumab-pegol-versus-adalimumab-2-year-efficacy-and-safety-results-from-a-superiority-investigator-blind-head-to-head-study/
