Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Assessment of the disease in psoriatic arthritis (PsA) remains a clinical challenge. Hence, there is no agreement on which index best correlates with the actual disease state of patients.
Objective: to assess the performance of and agreement between composite activity indices of common use in clinical practice (DAPSA and DAS66) and the physician global assessment (PhGA) to evaluate disease activity in PsA patients under biological therapy.
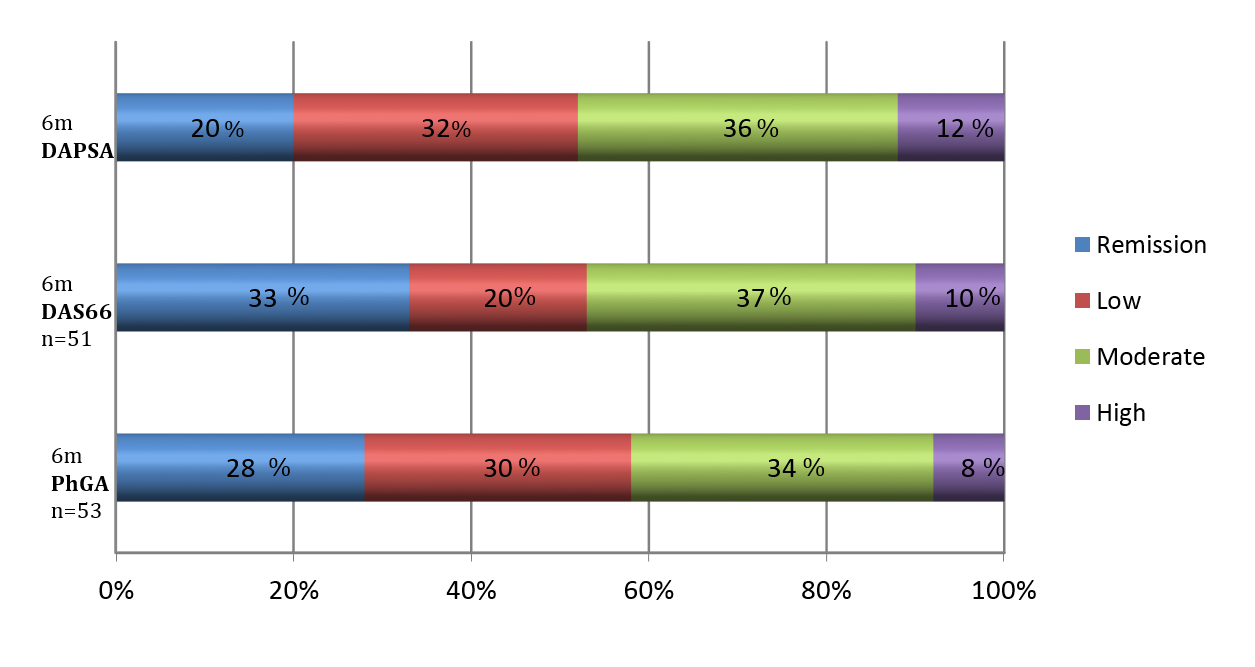
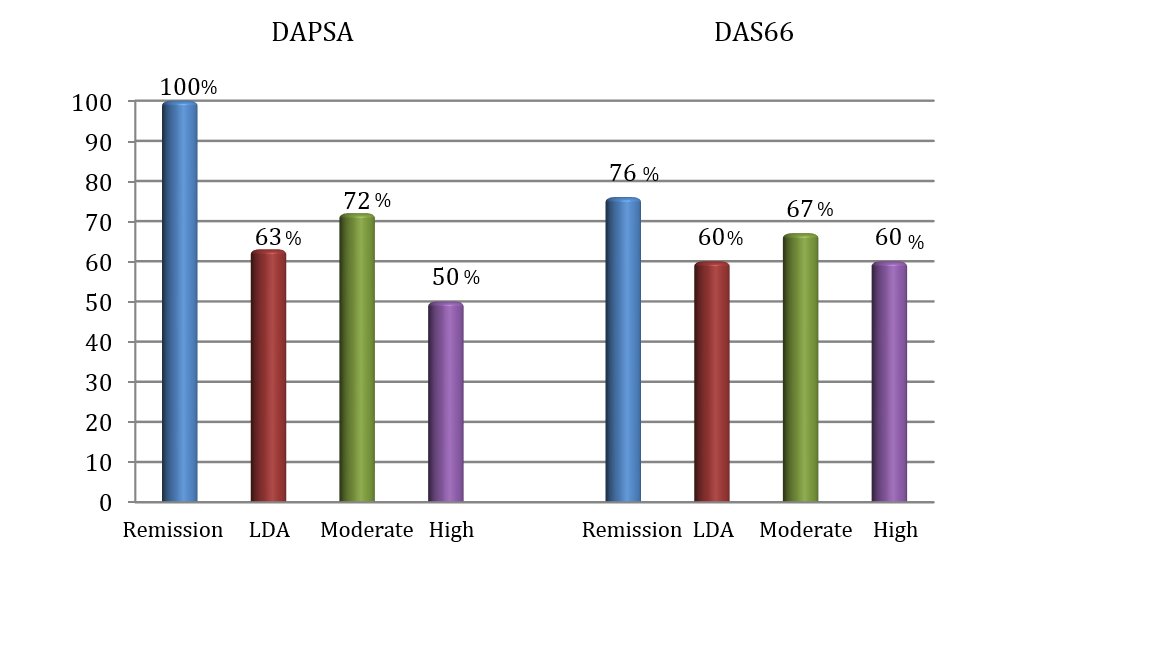
Methods: Patients with peripheral-only PsA initiating a first biological therapy (TNFi and IL-17i) between 2002 and 2018 in a university hospital were included. Demographic information, laboratory tests, concomitant treatments, PhGA and disease activity indexes (DAPSA and DAS66) were collected at baseline and 6 months (6 m) after starting biological treatment. At 6 m, the patients were classified in the following disease activity status: remission (DAPSA PhGA was also determined at 6 months, and it was assorted by consensus of 3 expert rheumatologists in remission (PhGA ≤ 10), LDA (PhGA >10-≤30), moderate activity (PhGA >30≤-60) and high activity (PhGA >60). The number (%) of patients achieving the different disease activity status by each of the different indexes at 6m and the agreement between PhGA scores and disease activity indices were determined.
Results: Out of 54 included patients, 20 (37%) were males, 37 (68%) were non-smokers, 34 (63%) had a BMI ≥25, with mean (SD) baseline disease activity indices of DAS 66: 4.8 (1.2) and DAPSA: 26 (14.9). Biological therapies initiated included etanercept in 37% of the cases, infliximab in 32%, adalimumab in 20%, golimumab in 4% and secukinumab in 7%. Clinical response at 6m according to different indexes and PhGA is depicted in Figure 1. More than half of the patients achieved at least LDA disease activity after 6 months of biological treatment, regardless of the index used. Thus, physician-perceived remission or LDA was frequent (58%). DAPSA index was the most stringent in terms of classifying patients in remission. Moderate to strong positive correlation was observed between DAS 66 and PhGA (Spearman r = 0.729, p < 0.001) and a strong correlation was observed between DAPSA and PhGA (Spearman r = 0.852, p < 0.001). The relative agreement between disease activity indexes and PhGA at 6 m for different disease activity states are shown in Figure 2. DAPSA classified better the patients in remission according to the PhGA.
Conclusion: In clinical practice, 50 % of the patients with PsA achieve at least a low activity state of the disease at 6 m after receiving a first biological therapy, and this is replicated by different indices. DAPSA based remission/LDA performed better than DAS66 to detect PhGA-defined remission or LDA.
To cite this abstract in AMA style:
Benavent D, Plasencia C, Navarro-Compán V, Peiteado D, Nuño L, Monjo I, Fernández E, Balsa A. Comparison Between Composite Activity Indices to Assess Clinical Response to Biological Therapy in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-between-composite-activity-indices-to-assess-clinical-response-to-biological-therapy-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-between-composite-activity-indices-to-assess-clinical-response-to-biological-therapy-in-patients-with-psoriatic-arthritis/