Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor under investigation for the treatment of psoriatic arthritis (PsA). Two Phase 3 studies have been completed (NCT01877668; NCT01882439) and a long-term extension (LTE) study is ongoing (database not locked; NCT01976364). This analysis compares incidence rates (IR) for adverse events (AEs) of special interest in a tofacitinib cohort from the Phase 3 PsA trials with real-world experience in a comparison cohort from the US Truven MarketScan database.
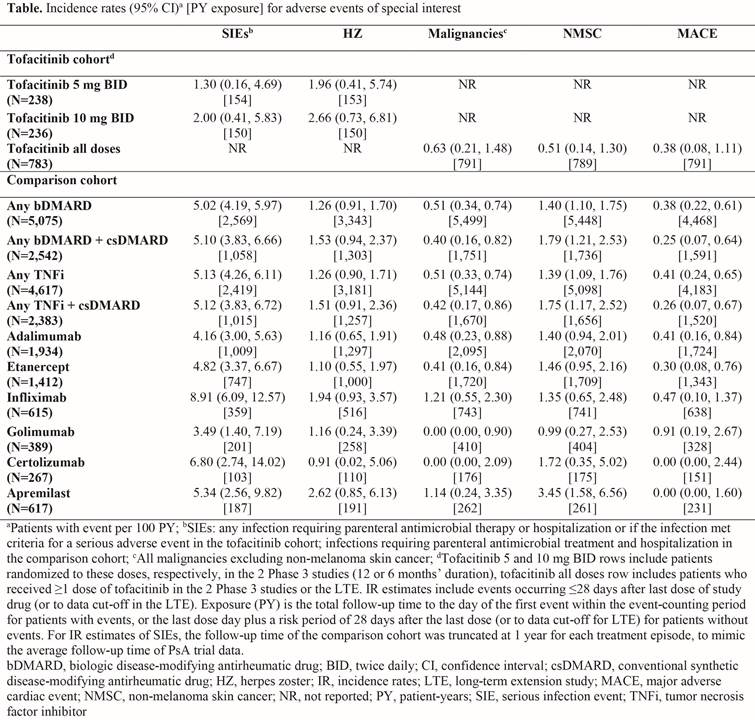
Methods: The tofacitinib cohort included adult patients (pts) from 2 Phase 3 studies with ≥6 months’ PsA diagnosis who met CASPAR criteria, had active plaque psoriasis, and active arthritis (≥3 swollen and ≥3 tender/painful joints). Pts were grouped by those who received tofacitinib 5 (N=238) or 10 mg (N=236) twice daily (BID) in the Phase 3 studies, and all pts who received ≥1 dose of tofacitinib in the Phase 3 studies or the LTE (tofacitinib all doses, N=783). The comparison cohort (N=5,799) comprised pts with moderate to severe PsA, defined by ≥1 inpatient or ≥2 outpatient 696.0 diagnosis codes on 2 unique calendar days (≥1 by a rheumatologist) between Oct 2010 and Sep 2015, or initiated therapy with a systemic agent for PsA. Key Phase 3 study exclusion criteria were applied to the comparison cohort. IRs for serious infection events (SIEs), herpes zoster (HZ), malignancies (excluding non-melanoma skin cancer [NMSC]), NMSC, and major adverse cardiovascular events (MACE) were compared.
Results: Mean age, gender, and diabetes history were generally similar between the tofacitinib and comparison cohorts (48.7–49.5 years, 42.4–49.2% male, 12.2–15.7% history of diabetes). Overall, more tofacitinib-treated pts had prior experience with corticosteroids (15.7–28.2%), csDMARDs (100%), and tumor necrosis factor inhibitors (48.1–55.9%) vs the comparison cohort (11.9%, 46.6%, and 36.6%, respectively). IRs per 100 PY (95% CI) for SIEs requiring parenteral antibiotics in an outpatient/emergency setting, or resulting in hospitalization were 1.30 (0.16, 4.69) and 2.00 (0.41, 5.83) in the tofacitinib 5 and 10 mg BID groups, respectively (Table). IRs were generally similar between the tofacitinib and comparison cohorts for SIEs resulting in hospitalization only, and for SIEs requiring parenteral antibiotics in emergency settings or resulting in hospitalization (data not shown). In general, the tofacitinib cohort had a higher rate of HZ vs the comparison cohort, and similar IRs for malignancies and MACE between cohorts (Table).
Conclusion: IRs of AEs of special interest reported in tofacitinib PsA Phase 3 studies were generally comparable to those in a general PsA population comprising pts receiving a range of biologic agents, except HZ, which was higher for tofacitinib-treated pts but similar to the incidence observed with tofacitinib treatment in other indications.
To cite this abstract in AMA style:
Curtis JR, Yun H, FitzGerald O, Winthrop K, Azevedo VF, Burmester GR, Rigby WFC, Kanik KS, Rojo R, Menon S, Wang C, Biswas P, Hendrikx T, Palmetto N. Comparing Tofacitinib Safety Profile in Patients with Psoriatic Arthritis in Clinical Studies with Real-World Data [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparing-tofacitinib-safety-profile-in-patients-with-psoriatic-arthritis-in-clinical-studies-with-real-world-data/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-tofacitinib-safety-profile-in-patients-with-psoriatic-arthritis-in-clinical-studies-with-real-world-data/