Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Various classes of biologic and targeted synthetic DMARDs (b/tsDMARDs) are now available for treatment of inflammatory arthritis (IA), but little information on their relative risk of side-effects exists from head-to-head comparisons in real-world settings. We aimed to compare the risk of severe infections associated with exposure to different classes of b/tsDMARDs for IA.
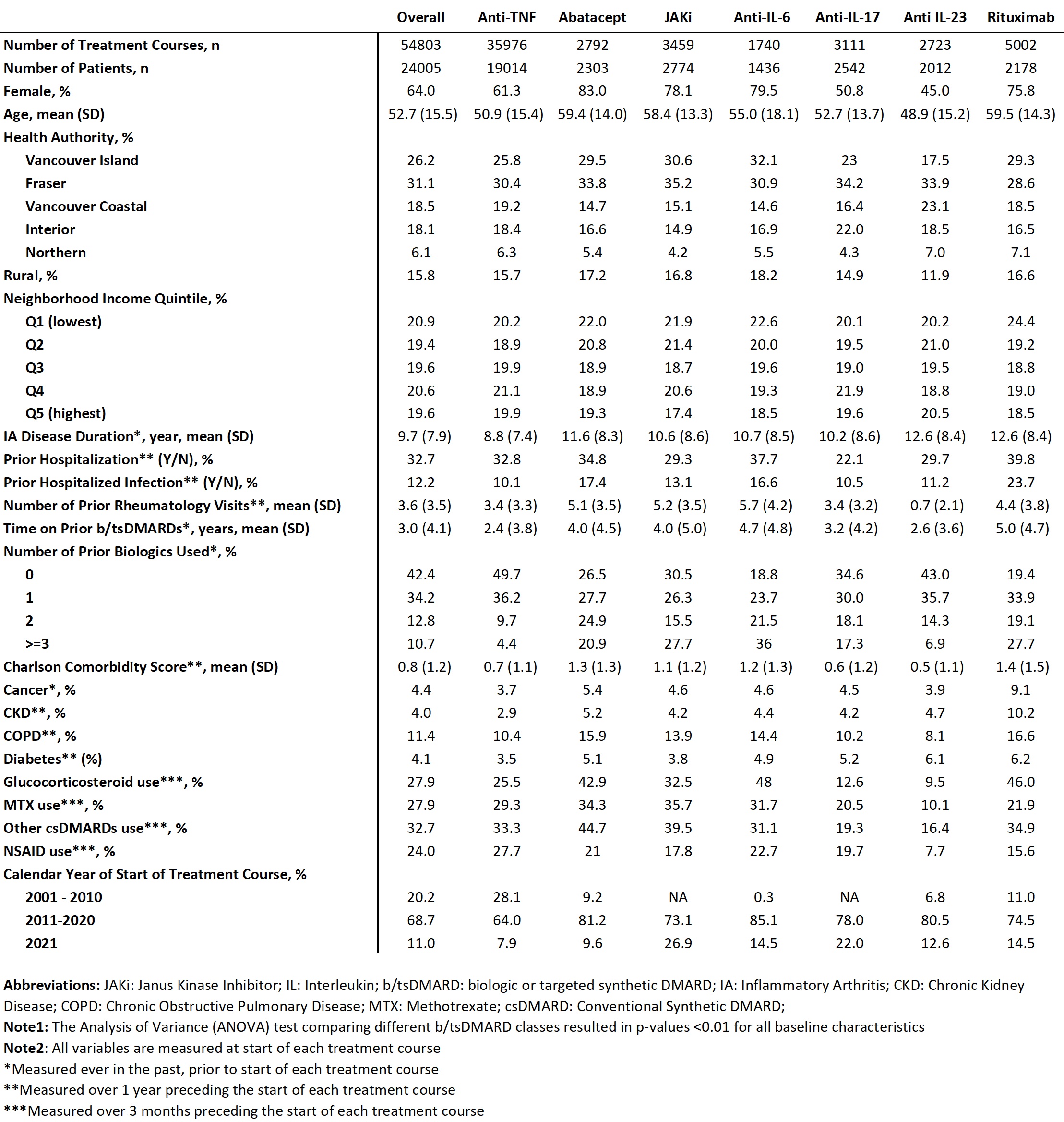
Methods: Design: Population-based cohort study, using administrative health data in a universal healthcare system. Sample: All incident b/tsDMARD users (i.e., without prescriptions over 5 yrs prior to initiating the first b/tsDMARD who started treatment between Jan 1996 and Dec 2021 for rheumatoid arthritis (RA), psoriasis or psoriatic arthritis (Pso/PsA), or ankylosing spondylitis (AS), followed until Dec 2022. People with non-Hodgkin’s lymphoma or chronic lymphocytic leukemia diagnosis 1 yr before or 6 mos after starting rituximab were excluded. Exposure was current use of b/tsDMARD categorized by class, with anti-TNFs as the reference.
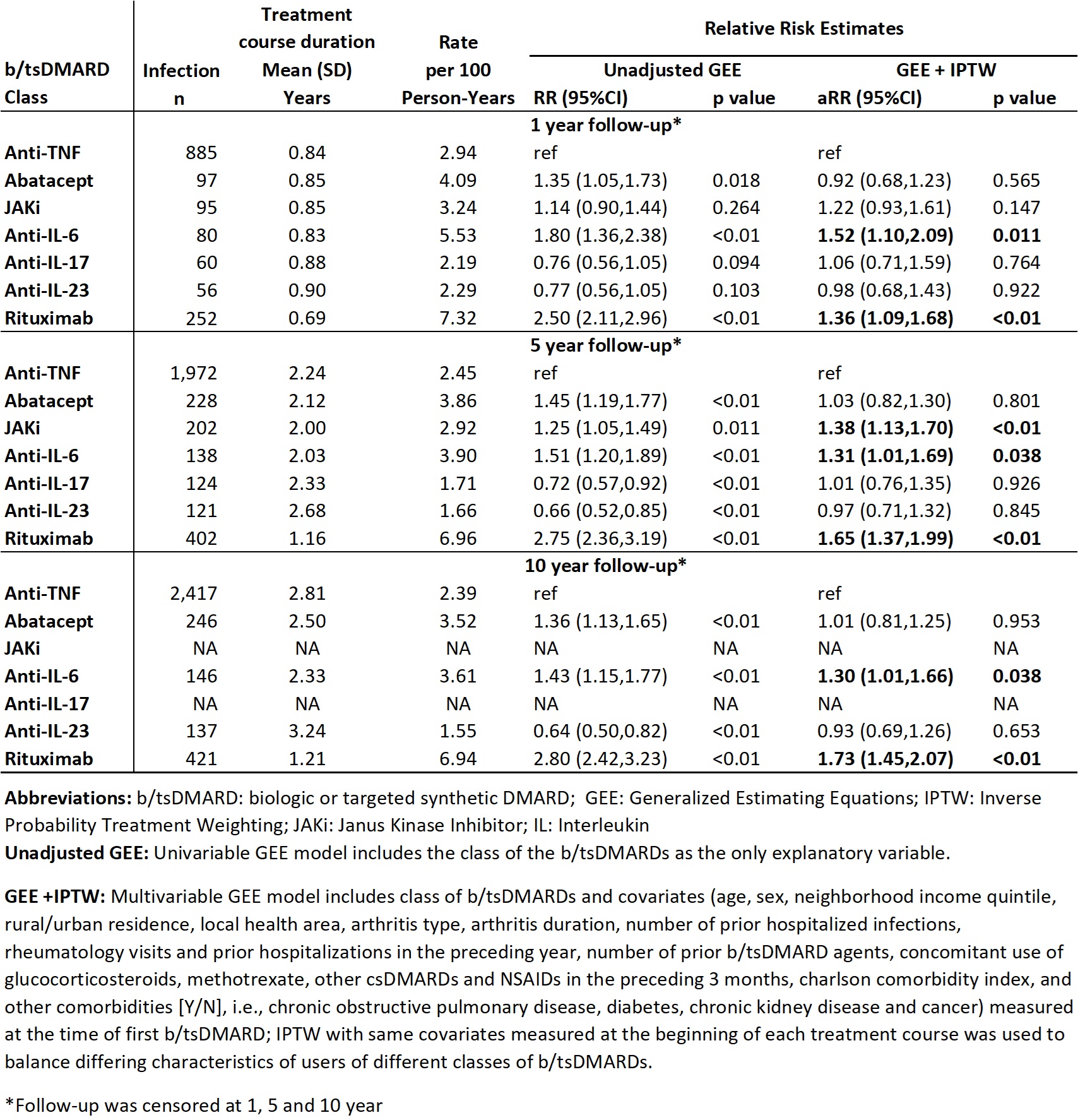
Unit of analysis was treatment course, starting from a new prescription for a b/tsDMARD, until discontinuation (defined as no renewal for ≥3 mos after prescription end date) or switching to another b/tsDMARD, plus a 90 day lag window (i.e. considered exposed for 90 days after discontinuation), or death, moving out of province or end of F/U, whichever occurred first. Drugs restarted after interruptions ≥3 mos were analysed as another treatment course for the same drug. The outcome was number of severe (hospitalized) infections identified by ICD-9/10 codes in any position in hospitalization data. Infection rates per 100 P-Y, rate ratios (RR) and 95% CI, at 1, 5 and 10 yrs of F/U (if available) were calculated. We conducted Marginal Structural Model analyses, using generalized estimating equation (GEE) models for repeated outcomes. To adjust for potential confounding due to differing characteristics of users of different b/tsDMARD classes, we used inverse probability of treatment weighting (IPTW) with covariates (listed in table 2) measured at start of each treatment course, and also adjusted for the same covariates at the time of first b/tsDMARD use in the GEE model.
Results: The sample includes 24,005 patients, followed for mean (SD) 6.2 (5.0) yrs after starting their first b/tsDMARD, with a total of 54,803 treatment courses (RA: 59%, PsA: 26%, AS: 15%), and a mean duration of treatment courses of 2.7 (3.2) yrs. See Table 1 for characteristics. After IPTW and adjustment, 1 yr risk of severe infections was significantly higher with anti-interleukin (IL) 6 (aRR 1.52; 95% CI 1.10, 2.09; p< 0.01) and rituximab (1.36; 1.09, 1.68; p< 0.01) relative to anti-TNF. The 5 yr risk was higher with Janus Kinase inhibitor (JAKi) (1.38; 1.13, 1.70; p< 0.01) as well as anti-IL6 and rituximab. The 10 yr results (when available) were similar (Table 2). No difference in risk was observed for abatacept, anti-IL17 and anti-IL23 relative to anti-TNF. Despite adjustments, residual confounding and channelling bias may persist.
Conclusion: Our population-based study provides real-world evidence suggesting that treatment with rituximab, JAKi and anti-IL6 is associated with a higher risk of severe infections relative to anti-TNFs for IA.
To cite this abstract in AMA style:
Moolooghy K, Xie H, Zheng Y, Avina-Zubieta J, Lacaille D. Comparing the Risk of Severe Infections Associated with Different Classes of Biologic or Targeted Synthetic Agents for Inflammatory Arthritis: A Population-based Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparing-the-risk-of-severe-infections-associated-with-different-classes-of-biologic-or-targeted-synthetic-agents-for-inflammatory-arthritis-a-population-based-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-the-risk-of-severe-infections-associated-with-different-classes-of-biologic-or-targeted-synthetic-agents-for-inflammatory-arthritis-a-population-based-study/