Session Information
Date: Tuesday, November 9, 2021
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II (1862–1888)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Posterior segment involvement is the most serious affection of uveitis in Behçet’s disease (BD), with cystoid macular edema (CME) being the leading cause of blindness. Anti-TNF, especially adalimumab (ADA) and infliximab (IFX), have demonstrated efficacy as first-line biologic agents in BD-related uveitis [Arthritis Rheumatol. 2019;71(12):2081-2089; Ophthalmology. 2018;125(9):1444-1451]. Moreover, the anti-IL6R tocilizumab (TCZ) has shown excellent results in highly refractory BD-uveitis and noninfectious uveitic CME [Rheumatology (Oxford). 2018;57(5):856-864; Am J Ophthalmol.2019;200:85-94; Clin Exp Rheumatol. 2014;32(4 Suppl 84): S54-7; Clin Exp Rheumatol. 2016;34(6 Suppl 102): S34-S40. Our aim was to compare the efficacy of ADA vs IFX vs TCZ in patients with refractory CME due to BD.
Methods: Observational multicenter study of patients with BD-associated CME refractory to conventional and/or biological immunosuppressive drugs. From a cohort of 177 patients treated with anti-TNF and 14 patients treated with TCZ, we selected those with CME at baseline. CME was defined as macular thickness > 300μm (measured by optic coherence tomography). We analyzed in the 3 groups of treatment (ADA, IFX, TCZ) from baseline up to 4 years the evolution of macular thickness (main outcome), best-corrected visual acuity (BCVA) and intraocular inflammation (Tyndall and vitritis). Differences between basal and final follow-up were evaluated. Multivariable linear regression was used to assess the differences between the 3 groups.
Results: A total of 49 patients were included. ADA was used in 25 patients (40 eyes with CME), IFX in 15 (21 eyes with CME) and TCZ in 9 (11 eyes with CME). Thus, a sum of 72 affected eyes was analyzed. No statistically significant baseline differences were observed between the 3 groups (Table 1) except for a lower basal BCVA in patients treated with TCZ and a higher basal degree of intraocular inflammation in patients treated with ADA. Most patients from all groups had received several conventional immunosuppressive drugs. In addition, most patients in the group of TCZ had also received anti-TNF (5 patients received ADA, 1 received IFX and 2 received both ADA and IFX, in different times). Biological therapy was used in monotherapy or combined with azathioprine (n=10, 5 and 1 in ADA, IFX and TCZ group, respectively), cyclosporine A (n=10, 5 and 1) or methotrexate (n=4, 2 and 3). Macular thickness progressively decreased in the 3 groups, with no signs of CME after 1 year of treatment (Figure 1). Similarly, BCVA improvement and inflammatory ocular remission was reached in all groups.
Conclusion: Refractory CME associated to BD’s uveitis can be effectively treated with ADA, IFX or TCZ. Moreover, TCZ is effective in patients resistant to anti-TNF therapy.
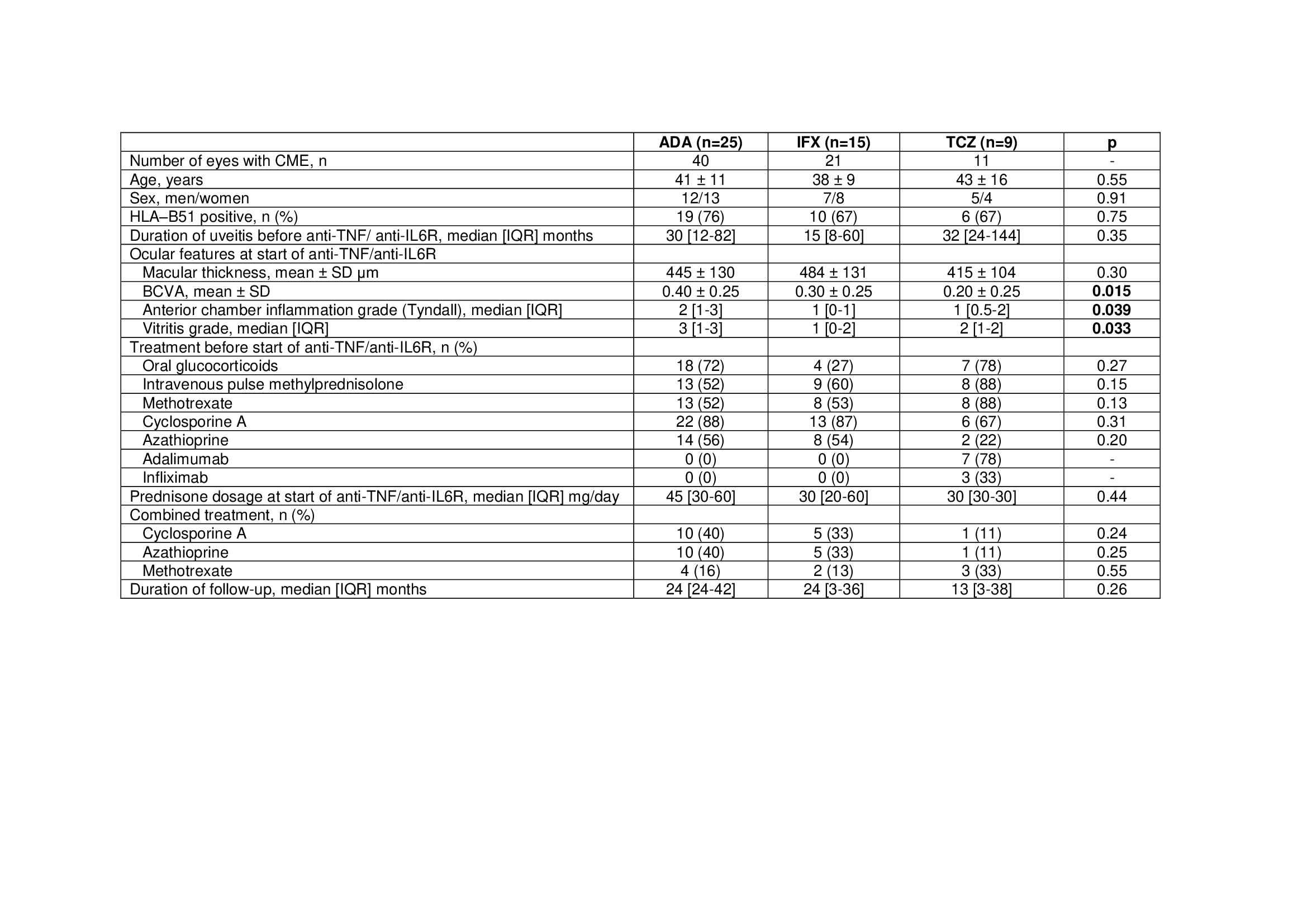 Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving ADA, IFX or TCZ. Data are presented as mean ± SD or median (IQR) when data were not normally distributed. ADA, adalimumab; BCVA, best corrected visual acuity; CME, cystic macular edema; IFX, infliximab; TCZ, tocilizumab.
Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving ADA, IFX or TCZ. Data are presented as mean ± SD or median (IQR) when data were not normally distributed. ADA, adalimumab; BCVA, best corrected visual acuity; CME, cystic macular edema; IFX, infliximab; TCZ, tocilizumab.
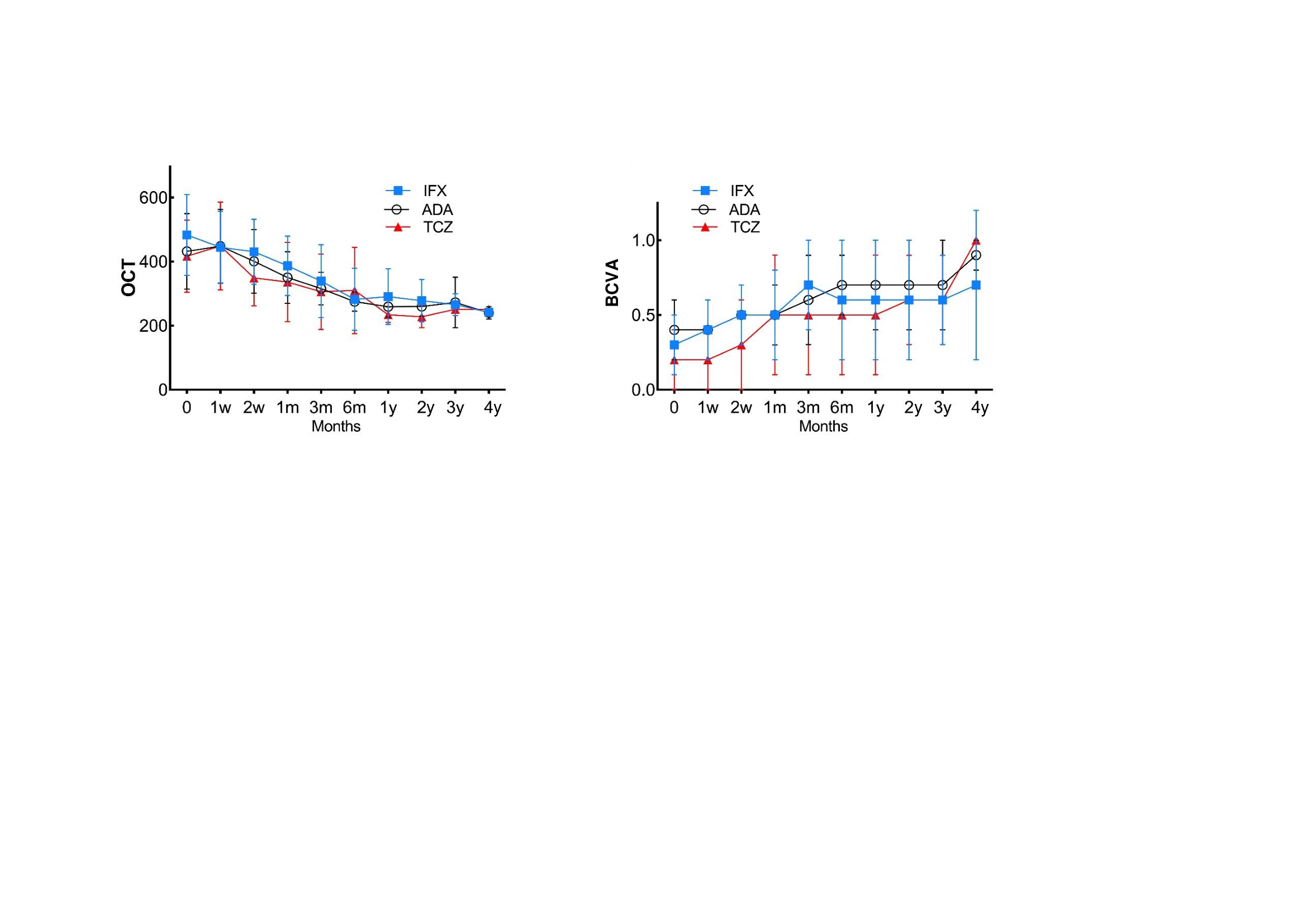 Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving ADA, IFX or TCZ.
Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving ADA, IFX or TCZ.
To cite this abstract in AMA style:
Atienza-Mateo B, Ferraz-Amaro I, Beltrán Catalán E, Adán A, Hernandez Garfella M, Martínez-Costa L, Cordero Coma M, Diaz-Llopis M, Herreras J, Blanco A, Torre I, Díaz-Valle D, Atanes-Sandoval A, Hernandez F, Insua S, Sánchez J, almodovar r, Ruiz-Moreno O, Gandia Martinez M, Nolla J, Martin-Varillas J, Calvo-Río V, Prieto-Peña D, gonzalez-Gay M, Blanco R. Comparative Study on Anti-TNF vs Tocilizumab for Treatment of Refractory Uveitic Cystoid Macular Edema Due to Behcet’s Disease: Multicenter Study of 49 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/comparative-study-on-anti-tnf-vs-tocilizumab-for-treatment-of-refractory-uveitic-cystoid-macular-edema-due-to-behcets-disease-multicenter-study-of-49-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-study-on-anti-tnf-vs-tocilizumab-for-treatment-of-refractory-uveitic-cystoid-macular-edema-due-to-behcets-disease-multicenter-study-of-49-patients/
