Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Belimumab, a biologic B-Lymphocyte stimulator (BLyS) inhibitor, was FDA-approved in 2011 for the treatment of active SLE. Initial phase 3 placebo-controlled trials found no increased risk of infection in patients initiated on belimumab in addition to standard therapy. However, the comparative risk of infection associated with initiating belimumab versus an oral immunosuppressant in non-renal SLE is unknown.
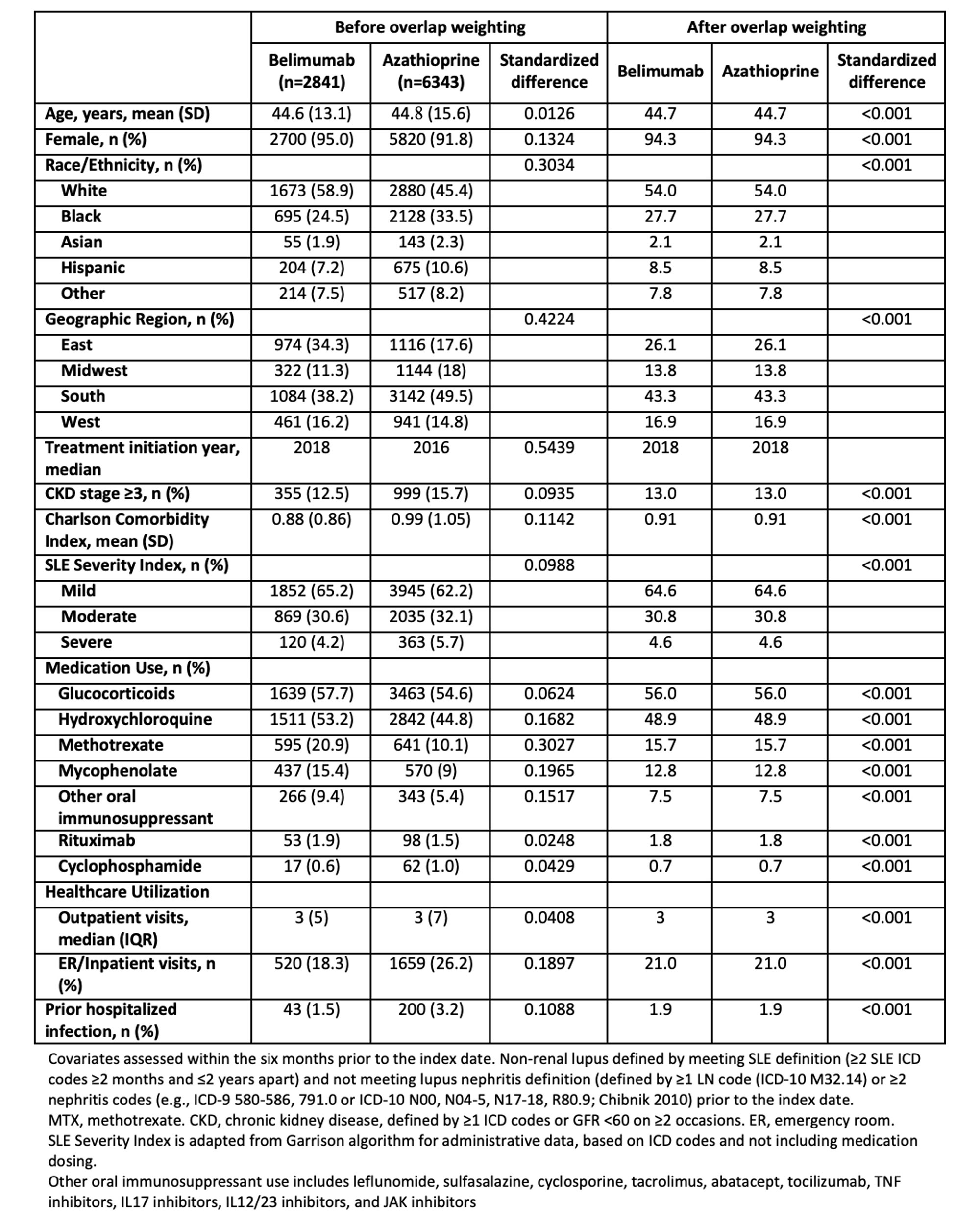
Methods: Using observational data from TriNetX, a multi-center electronic health record database including 46 health care organizations across the United States, we identified patients aged ≥18 with SLE (≥2 ICD codes ≥2 months and ≤2 years apart) who initiated belimumab, azathioprine, methotrexate, or mycophenolate between 2011-2021 and who did not have lupus nephritis (defined by ≥1 LN code (ICD-10 M32.14/15) or ≥2 nephritis codes (Chibnik 2010) prior to the index date. We designed and emulated three hypothetical target trials to estimate the cumulative incidence and hazard ratios (HRs) of severe infection and of hospitalization for severe infection comparing initiation of belimumab vs. azathioprine, belimumab vs. methotrexate, and belimumab vs. mycophenolate. In each comparison, patients had never used the comparators but could use other immunosuppressants (e.g., in belimumab vs. azathioprine comparison, methotrexate or mycophenolate could be used). In each analysis, we emulated randomization using propensity score overlap weighting to balance covariates, including age, sex, race/ethnicity, geographic region, year of initiation, use of concomitant SLE medications (other oral immunosuppressants, glucocorticoids, hydroxychloroquine, rituximab, cyclophosphamide), Charlson comorbidity index, SLE severity index (Garris 2013), chronic kidney disease, healthcare utilization, and prior infection history. Patients were followed until the outcome, death, or end of the study period, and we adjusted for adherence to treatment group using inverse probability of treatment weighting. We repeated the analysis with the negative control outcome of injury/trauma.
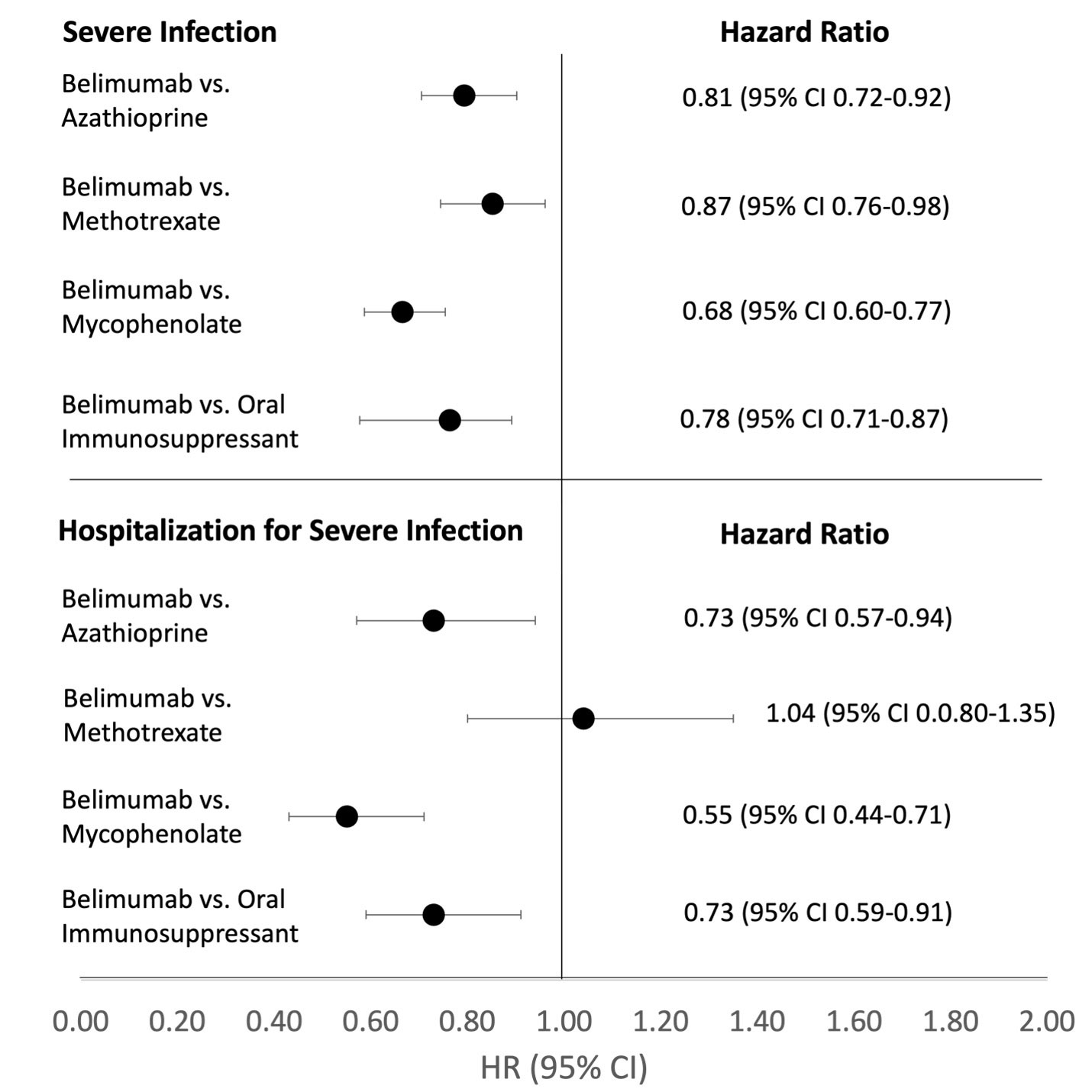
Results: We compared 2841 and 6343 initiators of belimumab and azathioprine (Table 1), 2642 and 8242 initiators of belimumab and methotrexate, and 2813 and 8407 initiators of belimumab and mycophenolate, respectively. After propensity score overlap weighting, all covariates were balanced in each comparison, with mean age 45 and 94% female; glucocorticoids were used by 56% of patients. Belimumab was associated with a lower incidence of severe infection (HR 0.81 [95% CI 0.72-0.92]) and hospitalization for infection (HR 0.73 [95% CI 0.57-0.94]) than was azathioprine through 5 years of use (Table 2). Findings were similar for the other medication comparisons (Figure 1). There was no difference in the risk of injury/trauma.
Conclusion: In this large cohort of patients with non-renal SLE, after rigorous propensity score overlap weighting to balance multiple covariates, belimumab was associated with a lower risk of severe infection and hospitalizations due to severe infection compared to several comparative oral immunosuppressants. This finding should inform risk/benefit considerations for SLE treatment.
To cite this abstract in AMA style:
Materne E, Choi H, Zhou B, Costenbader K, Zhang Y, Jorge A. Comparative Risks of Infection with Belimumab versus Oral Immunosuppressants in Patients with Non-Renal Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparative-risks-of-infection-with-belimumab-versus-oral-immunosuppressants-in-patients-with-non-renal-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risks-of-infection-with-belimumab-versus-oral-immunosuppressants-in-patients-with-non-renal-systemic-lupus-erythematosus/