Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: In randomized placebo-controlled trials, including the phase 4 Belimumab Assessment of Safety in SLE study, belimumab use was associated with a higher incidence of serious depression and suicidality than placebo. (Sheikh S et al, Lancet Rheumatol. 2020). However, the comparative risk of psychiatric outcomes between belimumab versus oral immunosuppressants is unknown.
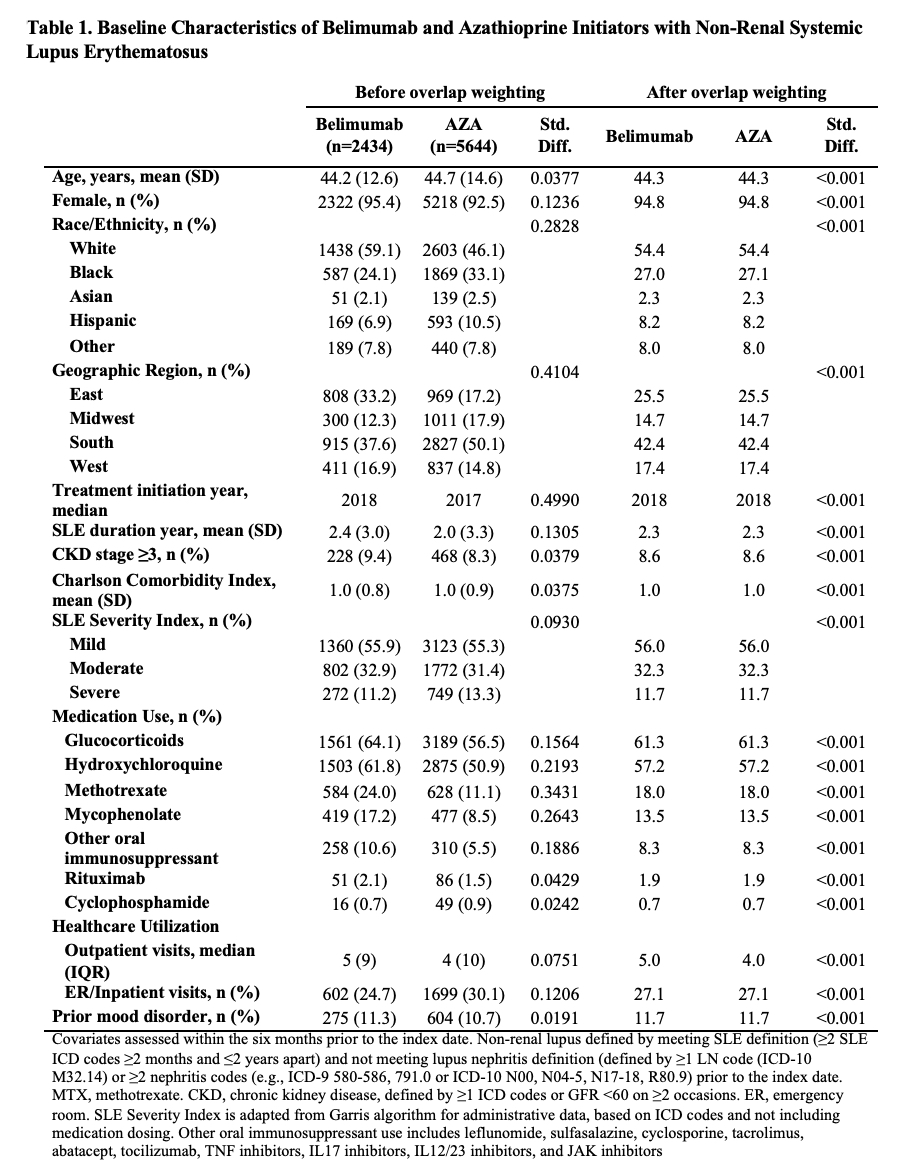
Methods: We conducted a comparative cohort study of adult patients with SLE who initiated treatment with belimumab versus an oral immunosuppressant (azathioprine [AZA], methotrexate [MTX], or mycophenolate [MMF]) between 2011-2021 using observational data from TriNetX, a United States multi-center electronic health records database. We excluded patients with lupus nephritis prior to the index date of treatment initiation. The outcomes of interest were hospitalization for depression and attempted suicide. We designed and emulated a hypothetical target trial to estimate the cumulative incidence and hazard ratios (HRs) of these outcomes associated with belimumab initiation versus each of the oral immunosuppressants. In three parallel analyses, we used propensity score overlap weighting to balance covariates, including age, sex, race/ethnicity, geographic region, year of initiation, use of concomitant SLE medications (glucocorticoids, hydroxychloroquine, rituximab, cyclophosphamide, other immunosuppressants), Charlson comorbidity index, SLE severity index, chronic kidney disease, healthcare utilization, and prior diagnoses of mood disorders. In a secondary population excluding patients with prior mood disorders, we also assessed the outcome of new onset depression. Patients were followed until the earliest of the outcome, death, end of the study period (August 31, 2021), or two years after treatment initiation. In a per-protocol analysis, patients were additionally censored for discontinuation of the assigned treatment or initiation of the comparator, and we adjusted for adherence to assigned treatment using inverse probability of treatment weighting.
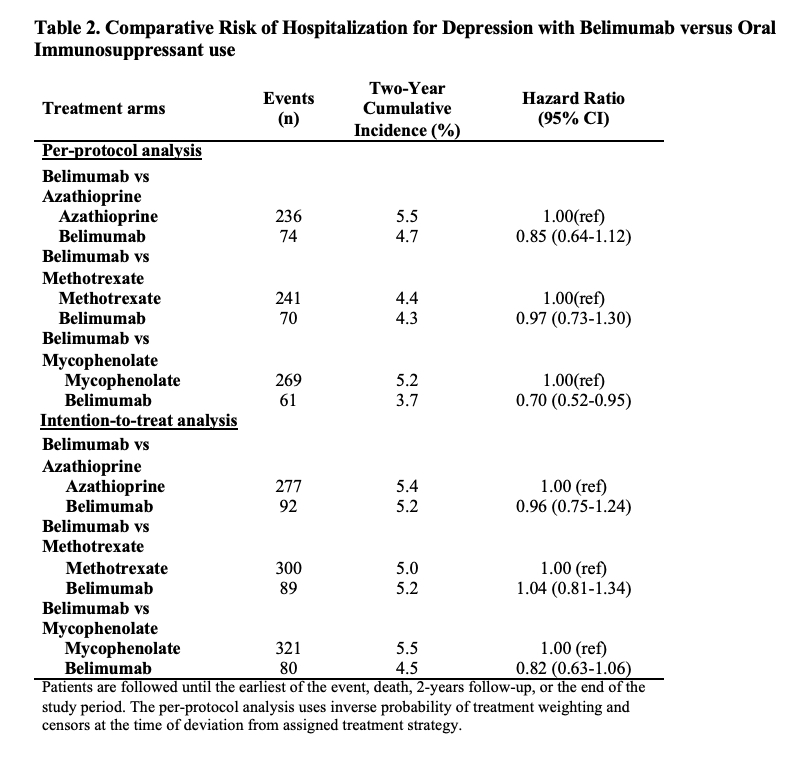
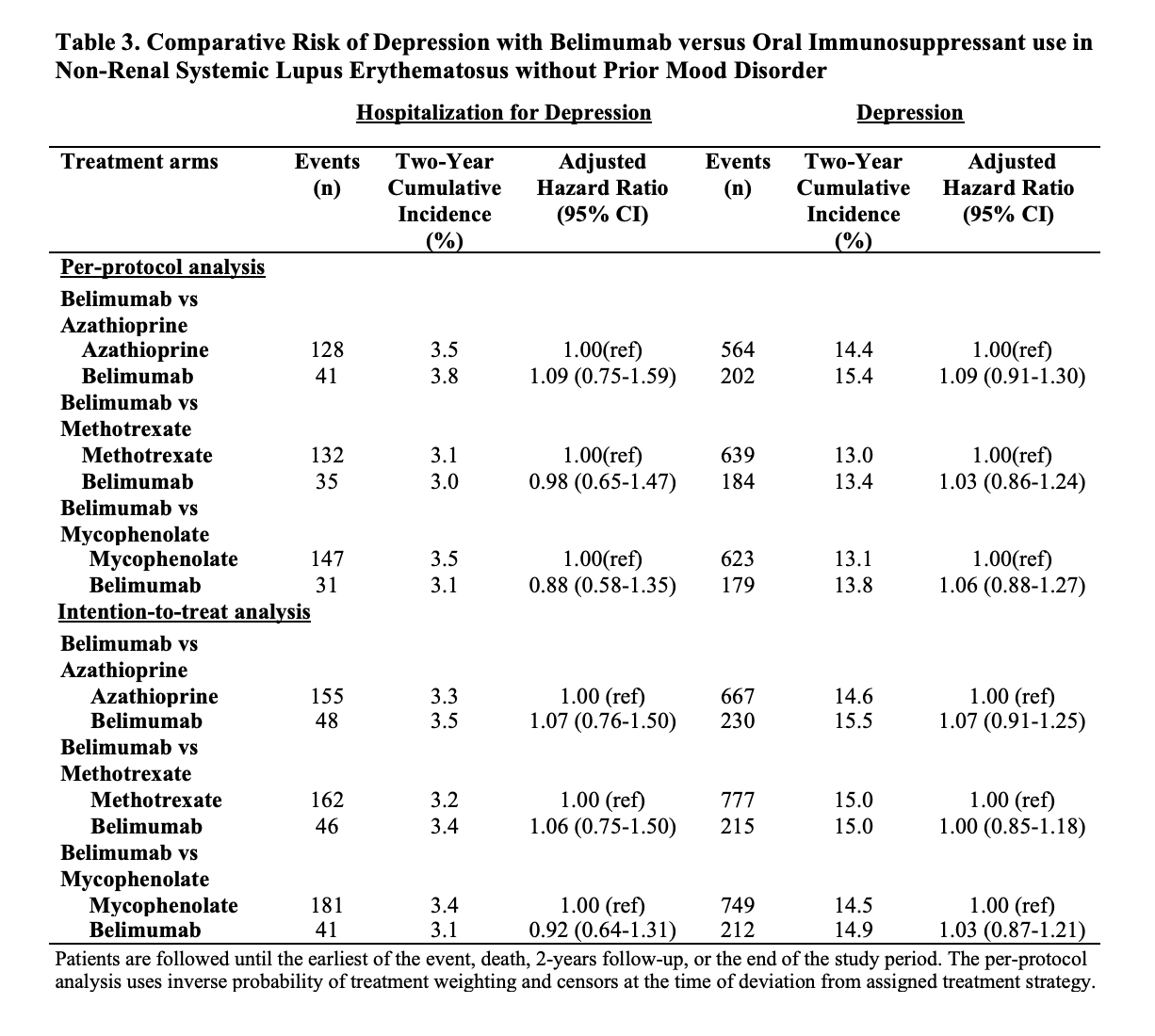
Results: Of 91,425 patients with SLE, we compared 2,434 and 5,644 initiators of belimumab and AZA, (Table 1), 2,163 and 7,224 initiators of belimumab and MTX, and 2,431 and 6,350 initiators of belimumab and MMF, respectively. After propensity score overlap weighting, covariates were balanced in each comparison. 95% were female; the mean age was 44 years. Attempted suicide was rare, with fewer than 10 events in each treatment group. There were no differences in the risk of hospitalized depression among belimumab initiators and initiators of AZA or MTX (Table 2). The risk of hospitalization for depression was lower for belimumab than MMF users (HR 0.70 [95% CI 0.52-0.95]). There was no difference in the risk of new onset depression among patients without a prior history of mood disorders (Table 3).
Conclusion: In this multi-center observational SLE cohort, we found that belimumab use was not associated with an increased risk of serious psychiatric events compared with oral immunosuppressants for SLE. This provides real-world reassurance of the safety of this medication for the treatment of SLE.
To cite this abstract in AMA style:
Jorge A, Zhou B, Zhang Y, Choi H. Comparative Risk of Serious Psychiatric Events with Belimumab versus Oral Immunosuppressant Use in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparative-risk-of-serious-psychiatric-events-with-belimumab-versus-oral-immunosuppressant-use-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-serious-psychiatric-events-with-belimumab-versus-oral-immunosuppressant-use-in-patients-with-systemic-lupus-erythematosus/