Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Opioid use for non-cancer pain has increased considerably over the last 30 years. The U.S. Food and Drug Administration announced several boxed warnings in 2016 to highlight serious opioid related risks in an effort to reduce fatal overdoses, 80% of which are unintentional. The most serious opioid related adverse event is respiratory depression (RD), which is potentially fatal. Few data exist on the incidence of RD in opioids users for non-malignant pain with no comparative data between drugs. The aim of this study was to assess the comparative risk of RD in new users of opioids for non-malignant pain using routinely collected electronic patient records (EPR).
Methods:
Opioid users from Salford hospital EPR were identified (26/9/14-31/5/16). Patients with prior malignancy were excluded using ICD-10 codes. Those with prior history of opioid use were excluded using keyword searches within the medicines reconciliation document. Administered medication was categorised as opioid monotherapy by drug or combination of opioids. Electronic National Early Warning Scores (regularly monitor vital signs during inpatient stays) were used to classify RD. An RD event was defined as any one of the following: respiratory rate (RR) ≤8/min, RR ≤10/min and oxygen saturations<94%, RR≤10/min and altered consciousness, or dispensed naloxone use. The primary analysis attributed RD to opioids during a risk window of ‘on drug + 1 day’, unless the patient switched to another opioid. Patients contributed follow up time for a particular drug from dispensed drug start date until day after discontinuation, first RD event, death or end of last hospital admission. Crude rates/1000 person years of follow up were calculated and Cox proportional hazards models were used to examine the comparative risk of administered opioids and RD, adjusted using propensity scores (PS) derived using inverse-probability of treatment weights.
Results:
7702 opioid users were included in the study, 3,839 female (50%) and a mean age (SD) of 52(21) years. There were 261 RD events observed on treatment, 130 with severe respiratory depression (RR<8/min), 135 requiring naloxone and 3 respiratory arrests. Patients on fentanyl, morphine, oxycodone and combination treatment had the highest crude rates (table). Patients on oxycodone had the highest proportion of comorbidities. In the propensity adjusted Cox-model, using codeine as the referent, patients on fentanyl, morphine, and combination opioids had the highest risk of RD (table). Compared to morphine, codeine had an adjusted HR of 0.64 (95% CI: 0.43, 0.95).
Conclusion:
Fentanyl and morphine monotherapy have a significantly higher risk of RD than codeine. Following PS adjustment the risk of RD on oxycodone no longer remained significant. The strengths of this study include real time physiological parameters to define RD and administered medication use (rather than prescribed) to define exposure.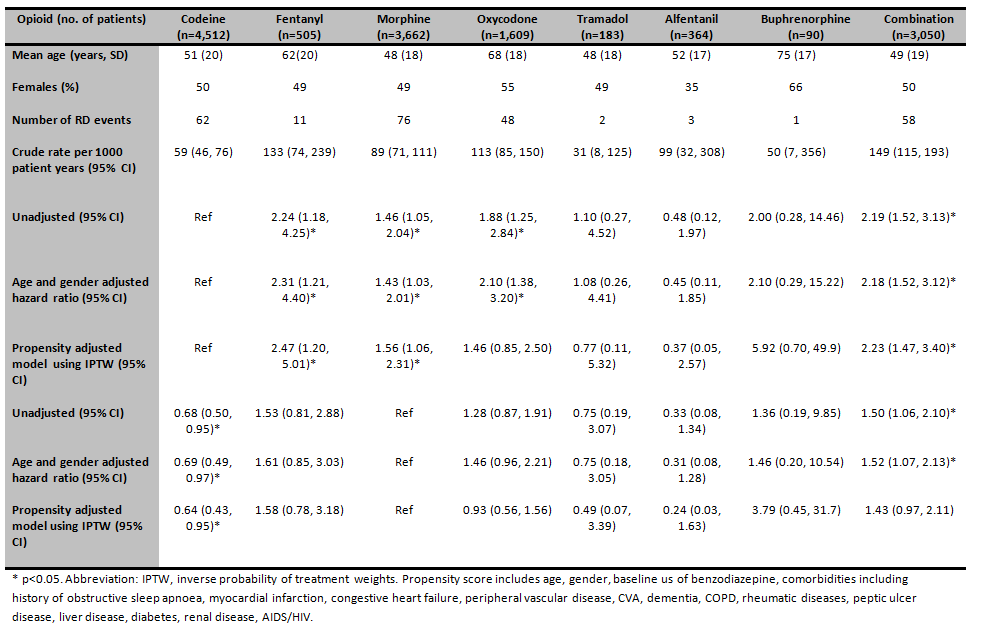
To cite this abstract in AMA style:
Jani M, Kopec-Harding K, Lunt M, Dixon WG. Comparative Risk of Respiratory Depression in Patients Treated with Opioids for Non-Malignant Pain [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparative-risk-of-respiratory-depression-in-patients-treated-with-opioids-for-non-malignant-pain/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-respiratory-depression-in-patients-treated-with-opioids-for-non-malignant-pain/
