Session Information
Date: Tuesday, November 7, 2017
Title: Plenary Session III
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Biologic DMARDs have varying mechanisms of action and may be associated with different infection risks. The perioperative time period is a particularly high-risk time for infections, which can carry significant morbidity. This study compared risk of post-operative infections after arthroplasty in patients with RA exposed to different biologic DMARDs or to methotrexate.
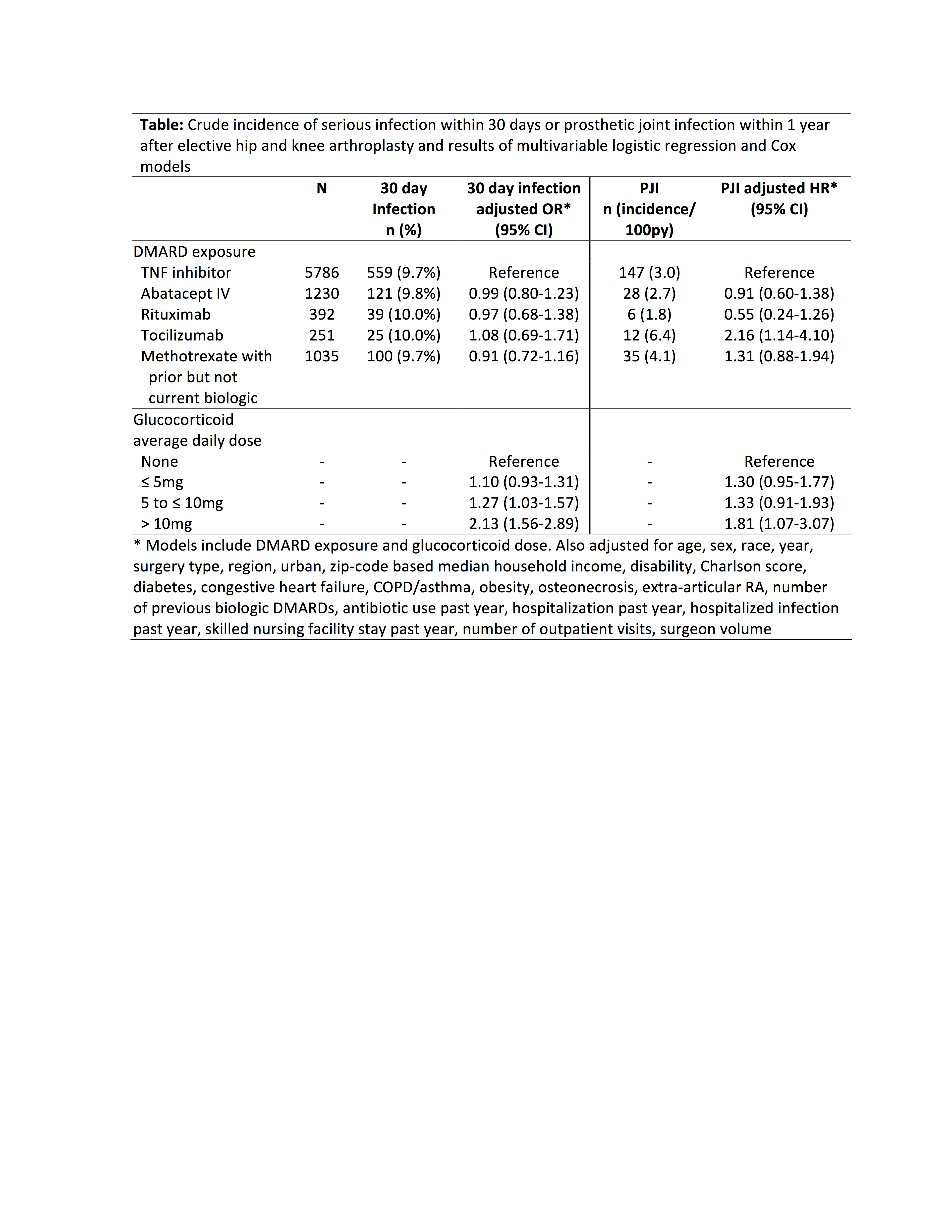
Methods: A retrospective cohort study using U.S. Medicare data from 2006-2014 evaluated adults with ≥ 2 ICD9 codes for RA undergoing elective inpatient primary or revision total knee or hip arthroplasty. Eligible patients received an infusion of infliximab, abatacept, or tocilizumab or prescription for adalimumab, or etanercept within 8 weeks, or a rituximab infusion within 16 weeks of surgery. Methotrexate treated patients with previous biologic treatment but no biologic within 6 months of surgery were also included. Patients with hip fracture, malignancy, pre-existing infection, or non-elective surgery (admission through the emergency department or as a hospital transfer) were excluded. Multivariable logistic or Cox regression evaluated associations between medication exposure and risk of 1) serious (hospitalized) infection within 30 days using a validated set of discharge diagnoses from inpatient discharge diagnoses and 2) rate of prosthetic joint infection (PJI, ICD9 996.66) within 1 year, adjusting for confounders.
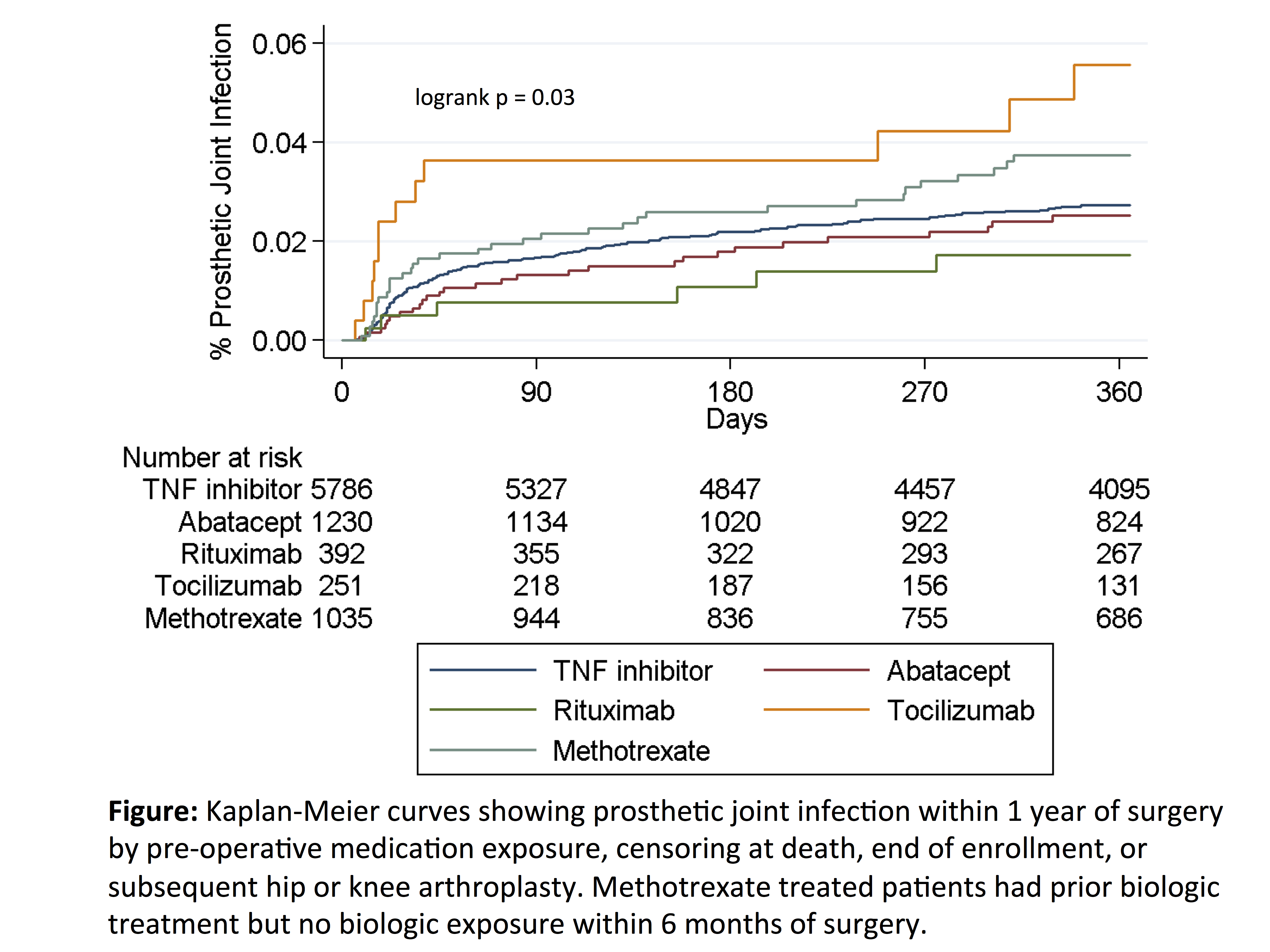
Results: Among 8694 surgeries in 7831 patients, serious infection occurred in 844 (9.7%), most commonly urinary infection, skin/soft tissue infection, and pneumonia. Infection incidence ranged from 9.7% to 10.0%, and risk was similar across medication exposure groups after adjustment for confounders (Table). Glucocorticoids were associated with a dose-dependent increase in risk, with significantly increased risk even at 5-10mg per day (Table). The overall rate of PJI within 12 months was 3.1/100 person-years (n = 228 infections), with crude rates ranging from 1.8 (rituximab) to 6.4 (tocilizumab) per 100 person-years (Figure). After adjustment, risk of PJI remained elevated in tocilizumab vs. TNF inhibitors [HR 2.16 (1.14-4.10), p = 0.02] (Table).
Conclusion: Short-term risk of serious post-operative infection after arthroplasty was similar in patients with RA treated with different biologics. Rates of PJI were low, with small variations in PJI risk between biologics possibly related to differences in disease severity. Glucocorticoids showed a strong dose-dependent association with infection, with a larger impact on post-operative infection risk than choice of biologic.
To cite this abstract in AMA style:
George MD, Baker J, Winthrop K, Alemao E, Chen L, Connolly S, Simon T, Wu Q, Xie F, Yang S, Curtis JR. Comparative Risk of Biologic Therapies in Patients with Rheumatoid Arthritis Undergoing Elective Arthroplasty [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparative-risk-of-biologic-therapies-in-patients-with-rheumatoid-arthritis-undergoing-elective-arthroplasty/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-biologic-therapies-in-patients-with-rheumatoid-arthritis-undergoing-elective-arthroplasty/