Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Recently published 2023 ACR/EULAR APS classification criteria emphasize delineating moderate/high anticardiolipin (aCL) and anti-β2 glycoprotein I (anti-β2GPI) antibodies. The new criteria specify the use of standardized ELISA methods for both aCL and anti-β2GPI with defined thresholds for moderate positivity (40-79 units) and high positivity (≥ 80 units). The exclusion of other solid phase aPL assay methods in new classification criteria is intriguing and justified by the absence of clinical research studies using automated test systems. We aimed to establish clinically important moderate/high thresholds for aCL and anti-β2GPI IgG/IgM chemiluminescent immunoassays (CLIA), in particular QUANTA Flash, comparable to our in-house ELISA thresholds used for over two decades, and to compare their diagnostic performance.
Methods: QUANTA Flash CLIA and in-house ELISAs were used to measure aCL and anti-β2GPI IgG/IgM. Moderate thresholds for QUANTA Flash CLIA were determined using a non-parametric approach, calculating a 99th percentile on serum samples from 139 blood donors and mirroring the diagnostic performance of in-house ELISA on 159 patient samples.
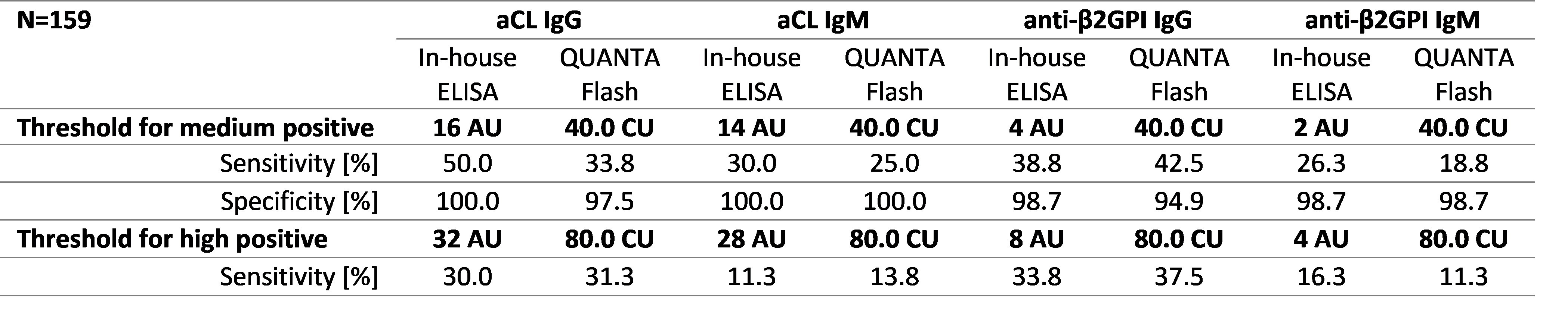
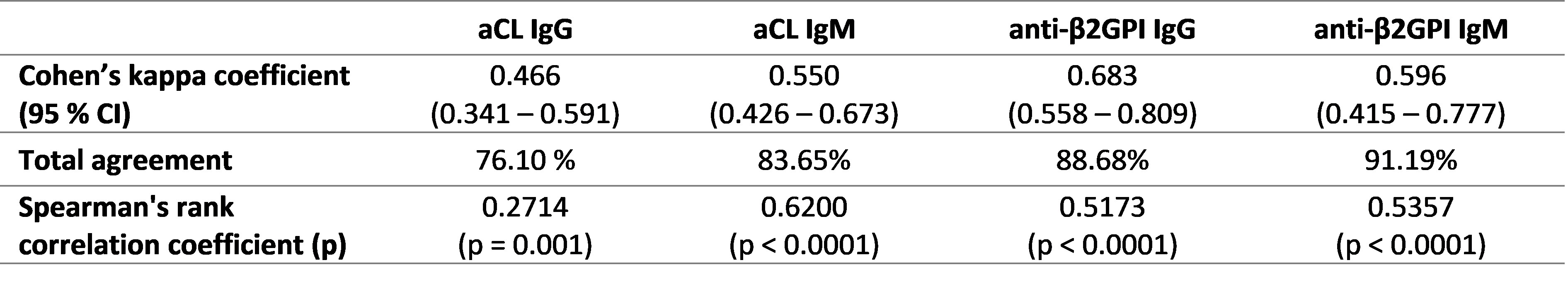
Results: Thresholds for QUANTA Flash CLIA achieving diagnostic performance comparable to in-house ELISAs were 40 CU for moderate and 80 CU for high levels for aCL and anti-β2GPI IgG and IgM (Table 1). The assays showed good qualitative agreement, ranging from 76.10 % to 91.19 % (Table 2).
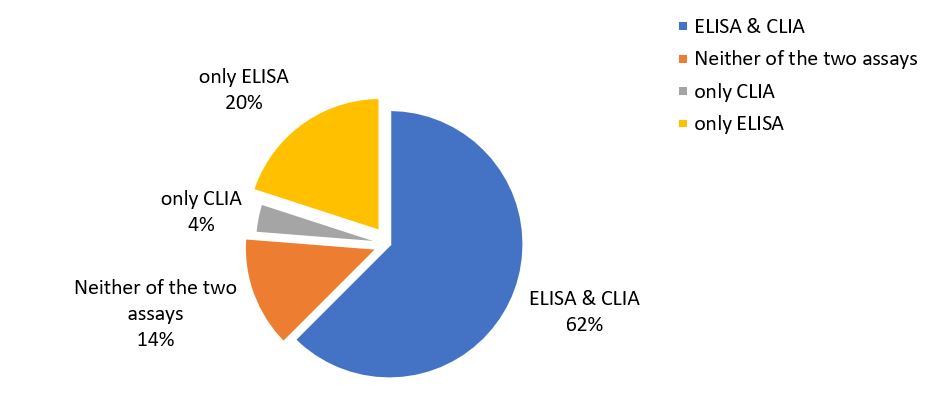
In our cohort of APS patients initially classified based on the revised Sapporo criteria, 50 of 80 (62.5%) were found to meet the new ACR/EULAR APS laboratory classification criteria, while 11 of 80 (13.8%) would not meet the criteria, regardless of the method used to determine aPL. The remaining 19 would fulfill the criteria according to one of the two methods, namely 3 according to CLIA and 16 according to ELISA (Figure 1).
In other words, it was found that 14 out of 80 (17.5%) patients would not fulfill the new ACR/EULAR APS laboratory classification criteria when considering only the in-house ELISA results, due to their single IgM positivity. However, among these patients, three individuals would meet the laboratory classification criteria when considering QUANTA Flash CLIA results. On the other hand, when evaluating the QUANTA Flash CLIA results, it was observed that 27 out of 80 patients (33.8%) would not meet the new ACR/EULAR APS laboratory classification criteria. However, among these patients, 16 individuals would meet the laboratory criteria when considering the in-house ELISA results.
Conclusion: We have identified relevant thresholds for aCL and anti-β2GPI IgG/IgM levels in QUANTA Flash CLIA, which is critical for the seamless integration of the new 2023 ACR/EULAR APS classification criteria into future observational clinical studies and trials. The in-house ELISA and QUANTA Flash CLIA showed good qualitative agreement. Notably, a significant proportion of APS patients classified according to the revised Sapporo criteria did not meet the new 2023 ACR/EULAR APS laboratory classification criteria (17% using ELISA vs. 33% using CLIA).
To cite this abstract in AMA style:
Zigon P, Bostič N, Rotar Z, Ambrozic A, Blokar E, ogrič M, Cucnik S. Comparative Performance of ELISA and CLIA Against the 2023 ACR/EULAR APS Classification Criteria [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-performance-of-elisa-and-clia-against-the-2023-acr-eular-aps-classification-criteria/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-performance-of-elisa-and-clia-against-the-2023-acr-eular-aps-classification-criteria/